
Concept explainers
(a)
Interpretation: The product formed when the
Concept introduction: The substitution reaction involves the replacement of one
Answer to Problem 20.43P
The product formed when the
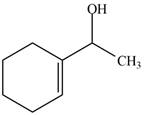
Figure 1
Explanation of Solution
The reaction that shows the formation of product when
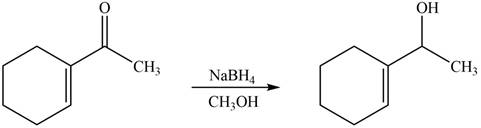
Figure 2
In the given reaction, sodium borohydride is used as a reducing agent. Sodium borohydride is used for the reduction of carbonyl compounds to alcohols.
The product formed when the
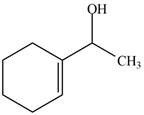
Figure 1
(b)
Interpretation: The product formed when the
Concept introduction: Addition of hydrogen takes place across a double bond of
Answer to Problem 20.43P
The product formed when the
Figure 3
Explanation of Solution
The reaction that shows the formation of product when
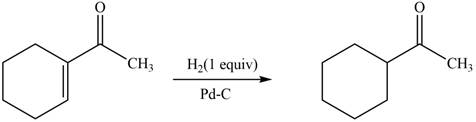
Figure 4
In the given reaction, hydrogenation of alkene takes place in the presence of palladium.
The product formed when the
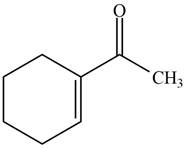
Figure 3
(c)
Interpretation: The product formed when the
Concept introduction: Addition of hydrogen takes place across a double bond of alkenes to form alkanes. This process is known as hydrogenation. It takes place in the presence of catalysts such as nickel, platinum or palladium.
Answer to Problem 20.43P
The product formed when the
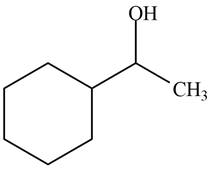
Figure 5
Explanation of Solution
The reaction that shows the formation of product when
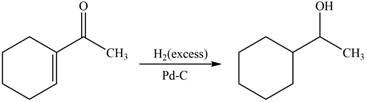
Figure 6
In the given reaction, excess hydrogen is used which will reduce both keto group and double bond. This results in the formation of a saturated compound.
The product formed when the

Figure 5
(d)
Interpretation: The product formed when the
Concept introduction: Methyllithium in the presence of water reduces carbonyl compounds into alcohol. Methyllithium is a reducing agent.
Answer to Problem 20.43P
The product formed when the
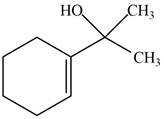
Figure 7
Explanation of Solution
The reaction that shows the formation of product when
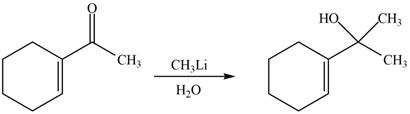
Figure 8
The product formed in the given reaction is
The product formed when the
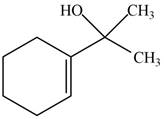
Figure 7
(e)
Interpretation: The product formed when the
Concept introduction: Grignard reagent is prepared by the reaction of alkyl or aryl bromide with magnesium metal in the presence of ether. The reaction of Grignard reagent with an
Answer to Problem 20.43P
The product formed when the
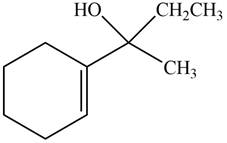
Figure 9
Explanation of Solution
The reaction that shows the formation of product when
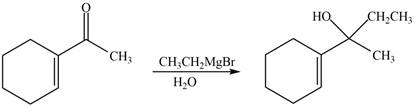
Figure 10
In the given reaction, Grignard reagent is used. This reagent attacks on the carbonyl compound to form alkoxides. In the next step, protonation with water takes place to give the final product.
The product formed when the
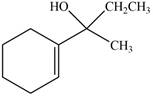
Figure 9
(f)
Interpretation: The product formed when the
Concept introduction: The steps involved in the reaction of ketone with organolithium reagent are as follows:
• The first step involves the reduction of carbonyl group into single bonds.
• The second step is the addition of R group from organometallic reagent and hydrogen to oxygen.
Answer to Problem 20.43P
The product formed when the
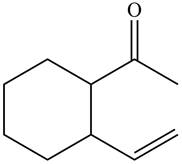
Figure 11
Explanation of Solution
The reaction that shows the formation of product when
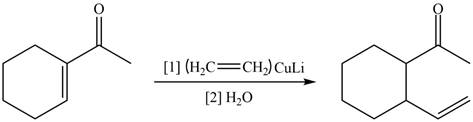
Figure 12
In the given reaction, organo copper reagent reacts with unsaturated carbonyl compound and forms carbon–carbon double bond with the beta carbon as shown in the above reaction.
The product formed when the

Figure 11
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry
- 1:14 PM Fri 20 Dec 67% Grade 7 CBE 03/12/2024 (OOW_7D 2024-25 Ms Sunita Harikesh) Activity Hi, Nimish. When you submit this form, the owner will see your name and email address. Teams Assignments * Required Camera Calendar Files ... More Skill: Advanced or complex data representation or interpretation. Vidya lit a candle and covered it with a glass. The candle burned for some time and then went off. She wanted to check whether the length of the candle would affect the time for which it burns. She performed the experiment again after changing something. Which of these would be the correct experimental setup for her to use? * (1 Point) She wanted to check whether the length of the candle would affect the time for which it burns. She performed the experiment again after changing something. Which of these would be the correct experimental setup for her to use? A Longer candle; No glass C B Longer candle; Longer glass D D B Longer candle; Same glass Same candle; Longer glassarrow_forwardBriefly describe the compounds called carboranes.arrow_forwardPlease don't use Ai solutionarrow_forward
- Pick the aromatic compound: A. 1,2,3 B. 1,2,4 C. 2,3,4 D. 1,3,4arrow_forwardNonearrow_forwardJON Determine the bund energy for UCI (in kJ/mol Hcl) using me balanced chemical equation and bund energies listed? का (My (9) +36/2(g)-(((3(g) + 3(g) A Hryn = -330. KJ bond energy и-н 432 bond bond C-1413 C=C 839 N-H 391 C=O 1010 S-H 363 б-н 467 02 498 N-N 160 N=N 243 418 C-C 341 C-0 358 C=C C-C 339 N-Br 243 Br-Br C-Br 274 193 614 (-1 214||(=olin (02) 799 C=N 615 AALarrow_forward
- Determine the bond energy for HCI ( in kJ/mol HCI) using he balanced cremiculequecticnand bund energles listed? also c double bond to N is 615, read numbets carefully please!!!! Determine the bund energy for UCI (in kJ/mol cl) using me balanced chemical equation and bund energies listed? 51 (My (9) +312(g)-73(g) + 3(g) =-330. KJ спод bond energy Hryn H-H bond band 432 C-1 413 C=C 839 NH 391 C=O 1010 S-1 343 6-H 02 498 N-N 160 467 N=N C-C 341 CL- 243 418 339 N-Br 243 C-O 358 Br-Br C=C C-Br 274 193 614 (-1 216 (=olin (02) 799 C=N 618arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardDon't used Ai solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





