
Draw the products formed when pentanal
a.
b.
c.
d.
e.
f.
g.
h.
i.
j.
k.
l. The [product in (a), then TBDMS-Cl, imidazole.
(a)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction: The substitution reaction involves the replacement of one functional group by other functional group. In nucleophilic substitution an electron rich species attack the species that is deficient in electrons. The electrophile and the leaving group together form a substrate. The nucleophile attacks over the substrate and there occurs the removal of leaving group from the substrate.
Answer to Problem 20.38P
The product formed by the reaction of pentanal with
![]()
Figure 1
Explanation of Solution
The reaction that shows the formation of product when pentanal reacts with
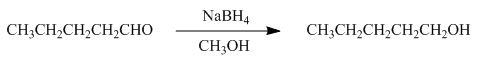
Figure 2
In the given reaction, sodium borohydride is used as a reducing agent. Sodium borohydride is used to for the reduction of carbonyl compounds to alcohols.
The product formed by the reaction of pentanal with
(b)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction: The substitution reaction involves the replacement of one functional group by other functional group. In nucleophilic substitution an electron rich species attack the species that is deficient in electrons. The electrophile and the leaving group together form a substrate. The nucleophile attacks over the substrate and there occurs the removal of leaving group from the substrate.
Answer to Problem 20.38P
The product formed by the reaction of pentanal with
![]()
Figure 1
Explanation of Solution
The reaction that shows the formation of product when pentanal reacts with
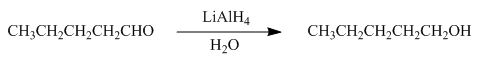
Figure 3
In the given reaction,
The product formed by the reaction of pentanal with
(c)
Interpretation: The product formed by the reaction of pentanal with the
Concept introduction: Addition of hydrogen takes place across a double bond of alkenes to form alkanes. This process is known as hydrogenation. It takes place in the presence of catalysts such as nickel, platinum or palladium.
Answer to Problem 20.38P
The product formed by the reaction of pentanal with the
![]()
Figure 1
Explanation of Solution
The reaction that shows the formation of product when
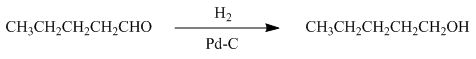
Figure 4
In the given reaction, hydrogenation of alkene takes place in the presence of palladium.
The product formed by the reaction of pentanal with the
(d)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction:
Answer to Problem 20.38P
No product is formed by the reaction of pentanal with
Explanation of Solution
The product formed by the reaction of pentanal with
![]()
Figure 5
No product is formed by the reaction of pentanal with
(e)
Interpretation: The product formed by the reaction of pentanal with the
Concept introduction: Organometallic reagents like
Answer to Problem 20.38P
The product formed by the reaction of pentanal with the
![]()
Figure 6
Explanation of Solution
The product formed by the reaction of pentanal with the
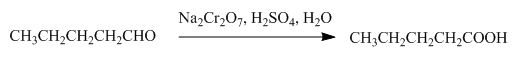
Figure 7
The product formed by the reaction of pentanal with the
(f)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction:
Answer to Problem 20.38P
The product formed by the reaction of pentanal with
![]()
Figure 6
Explanation of Solution
The product formed by the reaction of pentanal with
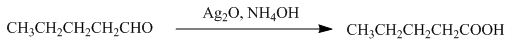
Figure 8
The product formed by the reaction of pentanal with
(g)
Interpretation: The product formed by the reaction of pentanal with the
Concept introduction: Grignard reagent is prepared by the reaction of alkyl or aryl bromide with magnesium metal in the presence of ether. The reaction of Grignard reagent with an aldehyde/ketone followed by hydrolysis yields an alcohol.
Answer to Problem 20.38P
The product formed by the reaction of pentanal with the
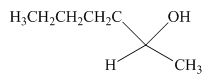
Figure 9
Explanation of Solution
The reaction that shows the formation of product when
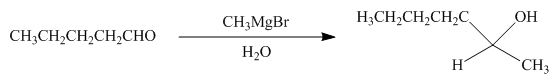
Figure 10
In the given reaction, Grignard reagent is used. This reagent attacks on the carbonyl compound to form alkoxides. In the next step, protonation with water takes place to give the final product.
The product formed by the reaction of pentanal with the
(h)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction: Organometallic reagents like
Answer to Problem 20.38P
The product formed by the reaction of pentanal with
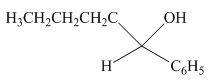
Figure 11
Explanation of Solution
The reaction that shows the formation of product when
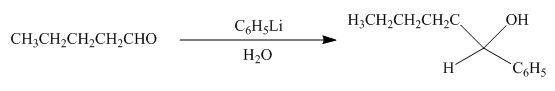
Figure 12
The product formed by the reaction of pentanal with
(i)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction: Organometallic reagents like
Answer to Problem 20.38P
No product formed by the reaction of pentanal with
Explanation of Solution
The product formed by the reaction of pentanal with
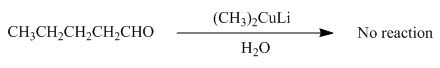
Figure 13
Aldehydes do not react with organo copper reagent because these reagents are less reactive and cannot attack to the electrophilic centre of the aldehyde group.
(j)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction: Organometallic reagents like
Answer to Problem 20.38P
The product formed by the reaction of pentanal with
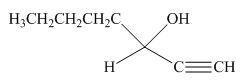
Figure 14
Explanation of Solution
The product formed by the reaction of pentanal with
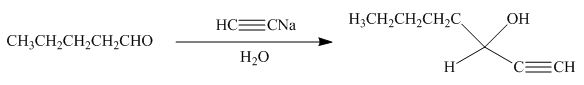
Figure 15
(k)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction: Organometallic reagents like
Answer to Problem 20.38P
The product formed by the reaction of pentanal with
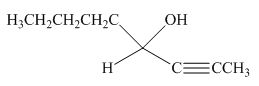
Figure 16
Explanation of Solution
The reaction that shows the formation of product when
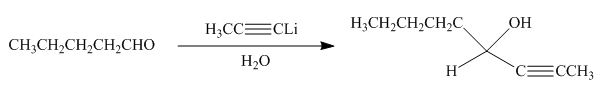
Figure 17
(l)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction: Organometallic reagents like
Answer to Problem 20.38P
The product formed by the reaction of pentanal with
Figure 18
Explanation of Solution
The product formed by the reaction of pentanal with
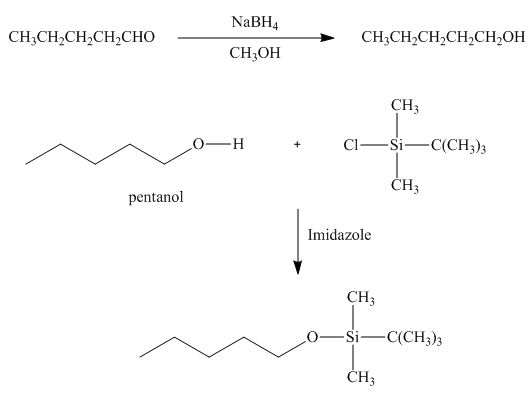
Figure 19
In the given reaction, sodium borohydride is used as a reducing agent. Sodium borohydride is used to for the reduction of carbonyl compounds to alcohols.
The product formed by the reaction of pentanal with the
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry
- this is an organic chemistry question please answer accordindly!! please post the solution draw the figures on a paper please hand drawn and post, please answer EACH part till the end and dont just provide wordy explanations, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forwardA mixture of 0.412 M C12, 0.544 M F2, and 0.843 M CIF is enclosed in a vessel and heated to 2500 K. C12(g) + F2(g )2CIF(g) Kc = 20.0 at 2500 K Calculate the equilibrium concentration of each gas at 2500 K. [C12] = M [F2] = M [ CIF] =arrow_forwardShow reaction mechanism with explanation. don't give Ai generated solutionarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
