
Concept explainers
(a)
Interpretation:
The structure of
Concept introduction:
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
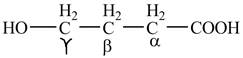
Explanation of Solution
The given compound contains four carbon atom hydrocarbon chain which is identified by butyric acid. It is also known as butanoic acid. Since its name ends with suffix
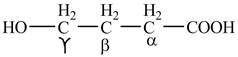
Figure 1
The structure of
(b)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
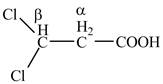
Explanation of Solution
The given compound contains three carbon atom hydrocarbon chain which is identified by propionic acid. It is also known as propanoic acid. Since its name ends with suffix
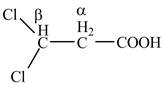
Figure 2
The structure of
(c)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
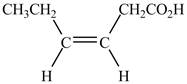
Explanation of Solution
The given compound contains six carbon atom hydrocarbon chain which is identified by hexenoic acid. It is an
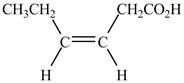
Figure 3
The structure of
(d)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
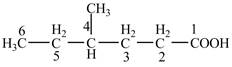
Explanation of Solution
The given compound contains six carbon atom hydrocarbon chain which is identified by hexanoic acid. Since its name ends with suffix
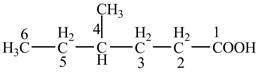
Figure 4
The structure of
(e)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
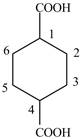
Explanation of Solution
The given compound contains six carbon atom hydrocarbon cyclic chain which is identified by cyclohexane. Since its name ends with dicarboxylic acid which means it contain two carboxyl groups and their position is
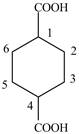
Figure 5
The structure of
(f)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
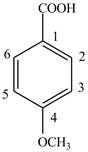
Explanation of Solution
The given compound contains six carbon atom
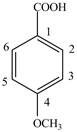
Figure 6
The structure of
(g)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
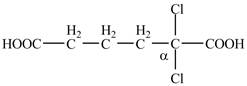
Explanation of Solution
The given compound contains six carbon atom hydrocarbon chain which is identified by adipic acid. Adipic acid is also known as
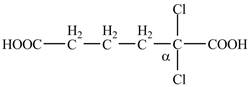
Figure 7
The structure of
(h)
Interpretation:
The structure of oxalic acid is to be drawn.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of oxalic acid is shown below.
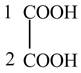
Explanation of Solution
The given compound contains two carbon atom hydrocarbon chain which is identified by oxalic acid. It is also known as
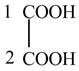
Figure 8
The structure of oxalic acid is shown in Figure 8.
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry
- Draw line structures of the following compounds and the product you would obtain from the reduction of each.(a) Isopropyl methyl ketone (b) p-Hydroxybenzaldehyde(c) 2-Methylcyclopentanonearrow_forwardDraw the structural formulas of the following compounds:(a) 2,3-Dimethylpentanal(b) 1,3-Dibromopropanone(c) 4-hydroxy-4-methylhexan-2-onearrow_forwardPlease draw the skeletal formula of the following compounds: (A) Isobutyraldehyde (B) α-Ethyl-γ-methoxycaproaldehyde (C) 6-Hydroxyhexanal (D) 2,4-Pentanedione (E)3-Cyano-7-oxoheptanoic acidarrow_forward
- In each of the following reactions, two possible organic products can be formed. Draw both organic products in each case and then circle the one formed in greatest quantity in each case. HC (a) 1) NaH, 2) acid (b) CH,CH,OH (c) CH,CH,OH NH2 (d) Oarrow_forwardGive the chemical tests to distinguish between following pair of compounds : (i) Propanol and propanone (ii) Ethyl acetate and methyl acetate (iii) Benzaldehyde and benzoic acid (iv) Benzaldehyde and acetaldehyde (v) Formic acid and acetic acid (vi) Propanal and propanol (vii) Ethanoic acid and ethylethanoatearrow_forward1. Draw structures corresponding to the following IUPAC names: (a) 4-Methylpentanoic acid (b) o-Hydroxybenzoic acid (c) 2,2-Dimethylpropanoyl chloride (d) trans-2-Methylcyclohexanecarboxamide (e) p-Methylbenzoic anhydride (f) p-Bromobenzonitrilearrow_forward
- Provide the correct structure from the IUPAC name. (a) 2-aminocyclopropanecarboxylic acid (b) 3-mercapto-5-oxoheptanal (c) Propyl 2,2,4-trimethyloct-4-enoate (d) 4-oxocycloheptanecarbaldehyde (e) N,N,4,4-tetraethylhexanamide (f) Acetic pentanoic anhydride (g) Acetyl chloride (h) Methyl 3,3-diethyl-6-octynoatearrow_forward(a) Explain how NaBH, in CH;OH can reduce hemiacetal A to 1,4-butanediol (HOCH,CH,CH,CH,OH). (b) What product is formed when A is treated with Ph;P=CHCH,CH(CH),? (c) The drug isotretinoin is formed by reaction of X and Y. What is the structure of isotretinoin? Although isotretinoin (trade name Accutane or Roaccutane) is used for the treatment of severe acne, it is dispensed under strict controls because it also causes birth defects. PPha NaOCH,CH3 HO- isotretinoin HO A Br X Yarrow_forwardWhich is the stronger acid in each of the following pairs? Explain your reasoning. (a) Phenol or p-hydroxybenzaldehyde (b) m-Cyanophenol or p-cyanophenol (c) o-Fluorophenol or p-fluorophenolarrow_forward
- Suggest a possible structure for Compound X.arrow_forwardWrite down the reaction of acetaldehyde with the following.(b) 2,4-dinitrophenylhydrazine (c) I2 / NaOHarrow_forwardGive reasons: (i) Bond length of C = O in carboxylic acids is slightly larger than C = O bond length in carbonyl compounds. (ii) There are two –NH2 groups in semicarbazide. However, only one –NH2 group is involved in the formation of semicarbazones. (iii) Benzoic acid is less soluble in water than acetic acid. (iv) Formic acid is a stronger acid than acetic acid.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





