
Concept explainers
(a)
Interpretation:
The structure of
Concept introduction:
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
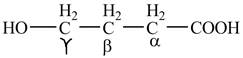
Explanation of Solution
The given compound contains four carbon atom hydrocarbon chain which is identified by butyric acid. It is also known as butanoic acid. Since its name ends with suffix
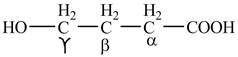
Figure 1
The structure of
(b)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
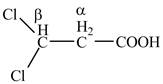
Explanation of Solution
The given compound contains three carbon atom hydrocarbon chain which is identified by propionic acid. It is also known as propanoic acid. Since its name ends with suffix
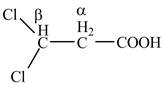
Figure 2
The structure of
(c)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
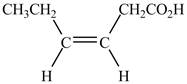
Explanation of Solution
The given compound contains six carbon atom hydrocarbon chain which is identified by hexenoic acid. It is an
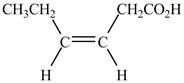
Figure 3
The structure of
(d)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
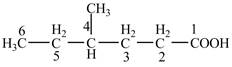
Explanation of Solution
The given compound contains six carbon atom hydrocarbon chain which is identified by hexanoic acid. Since its name ends with suffix
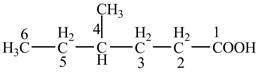
Figure 4
The structure of
(e)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
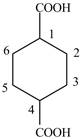
Explanation of Solution
The given compound contains six carbon atom hydrocarbon cyclic chain which is identified by cyclohexane. Since its name ends with dicarboxylic acid which means it contain two carboxyl groups and their position is
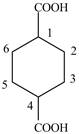
Figure 5
The structure of
(f)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
Explanation of Solution
The given compound contains six carbon atom
Figure 6
The structure of
(g)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
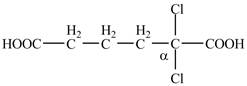
Explanation of Solution
The given compound contains six carbon atom hydrocarbon chain which is identified by adipic acid. Adipic acid is also known as
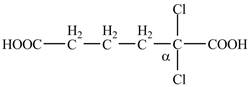
Figure 7
The structure of
(h)
Interpretation:
The structure of oxalic acid is to be drawn.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of oxalic acid is shown below.
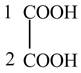
Explanation of Solution
The given compound contains two carbon atom hydrocarbon chain which is identified by oxalic acid. It is also known as
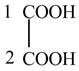
Figure 8
The structure of oxalic acid is shown in Figure 8.
Want to see more full solutions like this?
Chapter 20 Solutions
EBK ORGANIC CHEMISTRY
- (a) Draw the structure of the following :(i) p-Methylbenzaldehyde (ii) 4-Methylpent-3-en-2-one(b) Give chemical tests to distinguish between the following pairs of compounds :(i) Benzoic acid and Ethyl benzoate, (ii) Benzaldehyde and Acetophenone.(iii) Phenol and Benzoic acid.arrow_forwardDraw the structures of the following carboxylic acids.(a) a@methylbutyric acid (b) 2-bromobutanoic acid(c) 4-aminopentanoic acid (d) cis-4-phenylbut-2-enoic acidarrow_forwardGive reasons for the following :(i) Phenol is more acidic than methanol.(ii) The C—O—H bond angle in alcohols is slightly less than the tetrahedral angle (190°28′).(iii) (CH3)3C—O—CH3 on reaction with HI gives (CH3)3C—I and CH3—OH as the main products and not (CH3)3C—OH and CH3—I.arrow_forward
- Draw line structures of the following compounds and the product you would obtain from the reduction of each.(a) Isopropyl methyl ketone (b) p-Hydroxybenzaldehyde(c) 2-Methylcyclopentanonearrow_forwardPredict the major products formed when benzoyl chloride (PhCOCl) reacts with the following reagents.(a) ethanol (b) sodium acetate (c) anilinearrow_forwardDraw the structural formulas of the following compounds:(a) 2,3-Dimethylpentanal(b) 1,3-Dibromopropanone(c) 4-hydroxy-4-methylhexan-2-onearrow_forward
- 1. Draw structures corresponding to the following IUPAC names: (a) 4-Methylpentanoic acid (b) o-Hydroxybenzoic acid (c) 2,2-Dimethylpropanoyl chloride (d) trans-2-Methylcyclohexanecarboxamide (e) p-Methylbenzoic anhydride (f) p-Bromobenzonitrilearrow_forwardGive the chemical tests to distinguish between following pair of compounds : (i) Propanol and propanone (ii) Ethyl acetate and methyl acetate (iii) Benzaldehyde and benzoic acid (iv) Benzaldehyde and acetaldehyde (v) Formic acid and acetic acid (vi) Propanal and propanol (vii) Ethanoic acid and ethylethanoatearrow_forwardPredict the products (if any) of the following acid–base reactions.(a) acetic acid + ammoniaarrow_forward
- Compounds that contain an N-H group associate by hydrogen bonding. (a) Do you expect this association to be stronger or weaker than that of compounds containing an O-H group? (b) Based on your answer to part (a), which would you predict to have the higher boiling point, 1-butanol or 1-butanamine?arrow_forwardGiven that C6H11COOH has a pKa = 4.8 and C6H11N+H3 has a pKa = 10.7, (a) What pH would you make the water layer to cause the carboxylic acid to dissolve in the water layer and the amine to dissolve in the ether layer? (b) What pH would you make the water layer to cause the carboxylic acid to dissolve in the ether layer and the amine to dissolve in the water layer?arrow_forwardDraw the structure of each compound.(a) o-nitroanisole (b) 2,4-dimethoxyphenol (c) p-aminobenzoic acid(d) 4-nitroanilinearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





