
Biology 2e
2nd Edition
ISBN: 9781947172517
Author: Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 3VCQ
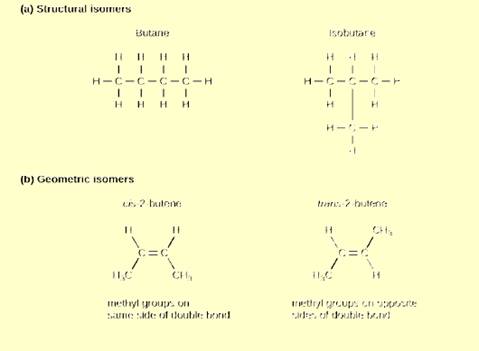
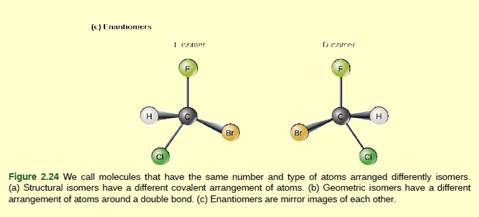
Figure 2.24 Which of the following statements is
false?
- Molecules with the formulas CH3CH2COOH and C3H6O2 could be structural isomers.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Structural isomers, like leucine and isoleucine, differ from each other in which of the following ways?
they have different numbers of atoms, but the same 3-dimensional arrangements of covalent bonds
they have different stoichiometric formulas, but the same number of covalent bonds
they have different atoms, but the same number of covalent bonds
they have different covalent bonds, but the same stoichiometric formulas
they have different numbers of atoms, and different 3-dimensional arrangements of covalent bonds
The dihedral or torsion angles of polypeptide backbone, the allowed values of which are summarized by the Ramachandran diagram, refer to rotation of the following pair of bonds.
Cα-N and Cα-R
C=O and Cα-N
C-N and Cα-C
Cα-C and Cα-N
Cα-R and Cα-C
The naturally-occurring amino acid L-serine (NH2-CH(COOH)-CH2-OH, dry form) shares all of the
following features with the naturally-occurring amino acid D-serine, except:
the same three-dimensional arrangement of covalent bonds
the same stoichiometry
the same number of covalent bonds
the exact same atoms
the same number of atoms
Chapter 2 Solutions
Biology 2e
Ch. 2 - Figure 2.3 How many neutrons do carbon-12 and...Ch. 2 - Figure 2.7 An atom may give, take, or share...Ch. 2 - Figure 2.24 Which of the following statements is...Ch. 2 - If xenon has an atomic number of 54 and a mass...Ch. 2 - Atoms that vary in the number of neutrons found in...Ch. 2 - Potassium has an atomic number of 19. What is its...Ch. 2 - Which type of bond represents a weak chemical...Ch. 2 - Which of the following statements is not true?...Ch. 2 - When acids are added to a solution, the pH should...Ch. 2 - We call a molecule that binds up excess hydrogen...
Ch. 2 - Which of the following statements is true? Acids...Ch. 2 - Each carbon molecule can bond with as many as...Ch. 2 - Which of the following is not a functional group...Ch. 2 - What makes ionic bonds different from covalent...Ch. 2 - Why are hydrogen bonds and van der Waals...Ch. 2 - Discuss how buffers help prevent drastic swings in...Ch. 2 - Why can some insects walk on water?Ch. 2 - What property of carbon makes it essential for...Ch. 2 - Compare and contrast saturated and unsaturated...
Additional Science Textbook Solutions
Find more solutions based on key concepts
The existence of the dwarf planet Pluto was proposed based on irregularities in Neptune's orbit. Pluto was subs...
College Physics
Pigeons may exhibit a checkered or plain color pattern. In a series of controlled matings, the following data w...
Concepts of Genetics (12th Edition)
Which type of cartilage is most plentiful in the adult body?
Anatomy & Physiology
In a mark-recapture study, an ecologist traps, marks, and releases 25 voles in a small wooded area. A week late...
Study Guide for Campbell Biology
QUANTITATIVE Punnett Squares as Genetic Tools. The genetic characters of seed color (where Y is the allele for ...
Becker's World of the Cell (9th Edition)
Compare and contrast aerobic respiration, anaerobic respiration, and fermentation.
Microbiology with Diseases by Body System (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- The “octet rule” in chemistry helps predict the types of bonds thatatoms will form. In general, an atom will be most stable if it fills itsouter shell of 8 electrons. Atoms with fewer than 4 valence electronstend to donate electrons and those with more than 4 valence electronstend to accept additional electrons; those with exactly 4 can do both.Using this rule, determine what category each of the followingelements falls into: N, S, C, P, O, H, Ca, Fe, and Mg. (You will needto work out the valence of the atoms.)arrow_forwardIn an ammonia molecule, one nitrogen atom (atomic number = 7; 1s² 2s²2p³) forms covalent bonds with three hydrogen atoms. Draw a diagram of an ammonia molecule. Show all valence electrons, lone pairs, molecular geometry, and partial charges (assume sp³ hybridization).arrow_forwardWhich of these statements about the tertiary structure of a polypeptide is(are) untrue? You may select more than one answer. The tertiary structure will be maintained when the solvent is changed from pure water to aqueous acetic acid. O The tertiary structure is determined in part by the formation of disulfide bridges. Hydrogen bonding interactions are responsible for formation of secondary structure but not tertiary structure, O The tertiary structure is rigid and unchanging. O Charge-charge (electrostatic) interactions can be important in the tertiary structure.arrow_forward
- :0-H-1--:N-H Hydrogen Bond Which statement best helps explain the formation of the hydrogen bond represented in the figure? A The oxygen has a partial positive charge, and the nitrogen has a partial negative charge. (B) The nitrogen has a partial negative charge, and the hydrogen attached to the oxygen has a partial positive charge.arrow_forwardExercise A: Amino Acid Functional Groups Figure 1 below shows one of the 20 amino acids that make up proteins. Recall that carbon can form four covalent bonds. Amino acids consist of a central carbon, called the a-carbon, that is bonded to four different chemical groups. H + CH2 OH Figure 1. Structure of an amino acid Answer the below questions in your own document. • On the amino acid shown in Figure 1, label the a-carbon. • The a-carbon of each of the 20 amino acids is bonded to one hydrogen atom, one amino group, one carboxyl group, and one R group (more on that below). You should recognize the amino and carboxyl groups from our discussion of functional groups in organic molecules. Circle and label* the amino group and the carboxyl group in Figure 1. *Note: our goal in this question, and in similar questions throughout this lab, is for you to be able to identify specific structures. You can do this circling/labeling in whatever way is easiest for you. You might want to draw the…arrow_forwardWhat are the names of the seven functional groups found in organic molecules? Underline the two that can act as an acid. Make the single group that acts as a base in bold lettering.arrow_forward
- The figure below illustrates the molecular structures of two fatty acids. A B H₂C The structural formula of erucic acid and behenic acid с H₂C D erucic acid behenic acid Which of the following best explains why erucic acid is liquid at room temperature but behenic acid is solid at room temperature? O OH The presence of a double carbon to carbon bond in erucic acid prevents the molecule from packing closely together. The lack of any double carbon-carbon bonds in behenic acid causes the molecule to be come polar and therefore packed more tightly. The larger number of carbon atoms in erucic acid prevents the molecule from packing tightly together. OH The smaller number of carbon atoms in behenic acid creates stronger covalent bonds between the carbon atoms allowing for them to pack more tightly together.arrow_forwardSuppose you had an organic molecule such as cysteine (see Figure 4.9,sulfhydryl group example), and you chemically removed the --NH2 group and replaced it with --COOH. Draw this structure. How would this change the chemical properties of the molecule? Is the central carbon asymmetric before the change? After?arrow_forwardwhich of the bonds in the molecule below are polar. explain why.arrow_forward
- Which of the following explains why methyl anion has a pyramidal geometry while methyl cation is planar? The HOMO energy decreases upon pyramidalization of the anion but increases for the cation Hyperconjugation stabilizes the planar cation Selected Steric repulsion between the H atoms disfavors the pyramidal anion All of the orbitals for the pyramidal structure are lower energyarrow_forwardC8H17NO2 is heated, and a condensation reaction occurs. A new molecule containing one peptide bond is formed and has the molecular formula C8H15NO. Draw the skeletal structure of this new compound.arrow_forwardThe carbohydrates (CHOs), with a general formula of (CH₂O)n, are rich in hydroxyl groups. This allows the carbohydrates incredibly diversity of structure and function. Match the structural feature with the functional feature allowed. CHOs are useful for fast energy production. CHOs are widely used to mediate molecular recognition events. CHOS are widely used as structural materials. 1. 2. 3. Hydroxyl groups can interact extremely well with water. Hydroxyl groups can be extensively derivatized and functionalized. Hydroxyl groups allow CHOs to form extensive H-bonded networks.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license