
Concept explainers
(a)
Interpretation:
The molecular formula of
Concept introduction:
The combustion is the process of heating the compounds in the presence of oxygen. The combustion of
Answer to Problem 2.45AP
The molecular formula of
Explanation of Solution
The moles of
Substitute the values in equation (1) for carbon as,
Substitute the values in equation (1) for hydrogen as,
The simplest ratio is the ratio of atomic ratio of carbon and hydrogen by atomic ratio of carbon. It is calculated to get the number of moles of element in the compound. The simplest ratio for carbon is as follows.
The simplest ratio for hydrogen is as follows.
Thus, the number of moles of
The formula obtained on multiplying the number of moles in
The molecular formula of
(b)
Interpretation:
The structures of the alkane obtained in (a) having two tertiary carbons and all other carbons secondary are to be drawn.
Concept introduction:
The carbons in a chemical formula may or may not be branched. The branched carbons are categorized as primary, secondary, tertiary, and quaternary carbons depending on the substituent attached to it. The branched carbons are categorized to understand the
The hydrogen’s attached to branched carbons are also categorized as primary, secondary and tertiary hydrogen’s.
Answer to Problem 2.45AP
The structures of the alkane obtained in (a) having two tertiary carbons and all other carbons secondary are shown below as,
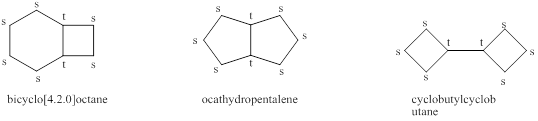
Explanation of Solution
A primary carbon is the one which is attached to only one carbon. Similarly, the secondary, tertiary, and quaternary carbons are attached to

Figure 1
The structures of the alkane obtained in (a) having two tertiary carbons and all other carbons secondary are shown in figure 1.
(c)
Interpretation:
The structure of the alkane obtained in (a) having no primary hydrogen’s, no tertiary carbon atoms, and one quaternary carbon atom is to be drawn.
Concept introduction:
The carbons in a chemical formula may or may not be branched. The branched carbons are categorized as primary, secondary, tertiary, and quaternary carbons depending on the substituent attached to it. The branched carbons are categorized to understand the chemical reactions in which these carbons are involved.
The hydrogen’s attached to branched carbons are also categorized as primary, secondary and tertiary hydrogen’s.
Answer to Problem 2.45AP
The structure of the alkane obtained in (a) having no primary hydrogen’s, no tertiary carbon atoms, and one quaternary carbon atom is shown below as,
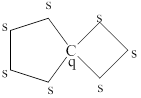
Explanation of Solution
A primary carbon is the one which is attached to only one carbon. Similarly, the secondary, tertiary, and quaternary carbons are attached to

Figure 2
The structure of the alkane obtained in (a) having no primary hydrogen’s, no tertiary carbon atoms, and one quaternary carbon atom is shown in figure 2.
Want to see more full solutions like this?
Chapter 2 Solutions
Organic Chemistry
- Please answer letters a and barrow_forwardCyclopropane (C3H6, a three-membered ring) is more reactive than most other cycloalkanes.(a) Draw a Lewis structure for cyclopropane.(b) Compare the bond angles of the carbon atoms in cyclopropane with those in an acyclic (noncyclic) alkane.(c) Suggest why cyclopropane is so reactive.arrow_forward2.25 A certain hydrocarbon has a molecular formula of C5 Hg. Which of the following is not a structural possibility for this hydrocarbon? (a) It is a cycloalkane. (b) It contains one ring and one double bond. (c) It contains two double bonds and no rings. (d) It is an alkyne.arrow_forward
- Pentane and pentene. (a) Are isomers because they have the same molecular formula. (b) Are isomers because they have the same number of carbon atoms. (c) Are not isomers because they have different molecular formulas (d) Are not isomers because they have different namesarrow_forward(c) There is one (1) other positional isomer of X missing for reaction II. Draw the structural formula of this isomer.arrow_forward(a) Calculate the standard enthalpy change for the combustion of 1 mol of benzene, C6H61l2, to CO21g2 and H2O1l2.(b) Compare the quantity of heat produced by combustion of 1.00 g propane with that produced by 1.00 g benzene.arrow_forward
- Among the four alkanes, ethane, propane, butane, and pentane, which is capable of existing in isomeric forms?arrow_forwardClassify each of he following hydrocarbons as alkanes, alkenes, or alkynes. (a) C6H14 (b) C3H4 (c) C9H18arrow_forward1.) How does an alkane structurally differ from an alkene?arrow_forward
- (b) H₂C 4-ethy-3-methylnonane CH₂ Marvin JS H₂ X Write the structural formulas for the following hydrocarbons. (c) 2,2-dimethylbutane Helparrow_forwardThe skeletal line formula for a branched alkene is shown below. (i) What is the molecular formula of this compound? (ii) How many carbon atoms are in the longest chain, ignoring the double bond? (iii) What is the longest chain incorporating both carbons of the double bond? (iv) How many substituents are on this chain? (v) Give the IUPAC name for this compound. [6]arrow_forward5. There are several classes of organic compounds commonly referred to as organic families, and categorized based on bonding patterns. These include: aromatics, esters, alkanes, alcohols, alkynes and alkenes. Which of these is/are considered to be (an) unsaturated hydrocarbon(s)? Provide a brief but explanation for your answer.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

