
Where on the blank outline of the periodic table do elements that meet the following descriptions appear?
- (a) Elements with the valence-shell ground-state electron configuration ns2 np5
- (b) An element whose fourth shell contains two p electrons
- (c) An element with the ground-state electron configuration [Ar] 4s2 3d10 4p5
(a)
Interpretation:
Elements with the given valence shell electronic configuration has to be identified.
Explanation of Solution
Given valence shell electronic configuration,
Periodic table is given in figure 1.
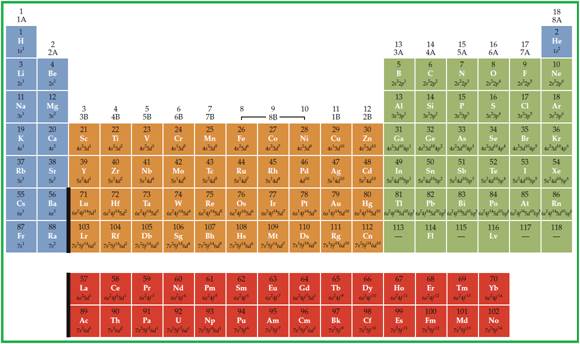
Figure 1
The elements with the given valence shell electronic configuration are highlighted (Red color) in the below periodic table
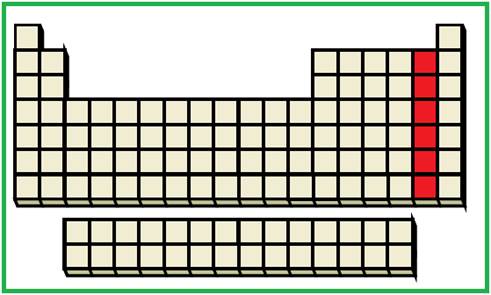
Figure 2
(b)
Interpretation:
An element with fourth shell containing two
Explanation of Solution
Given valence shell electronic configuration,
Periodic table is given in figure 1.

Figure 1
An element with fourth shell contains two
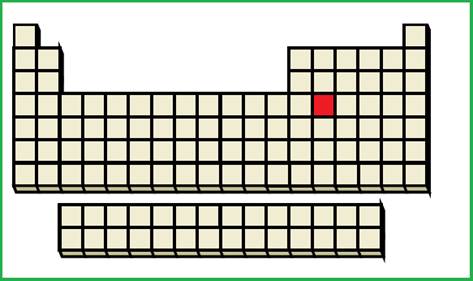
Figure 3
(c)
Interpretation:
An element with given ground-state electronic configuration has to be identified.
Explanation of Solution
Given ground-state electronic configuration,
Calculate the total number of electrons
Periodic table is given in figure 1.

Figure 1
An element with fourth shell contains two
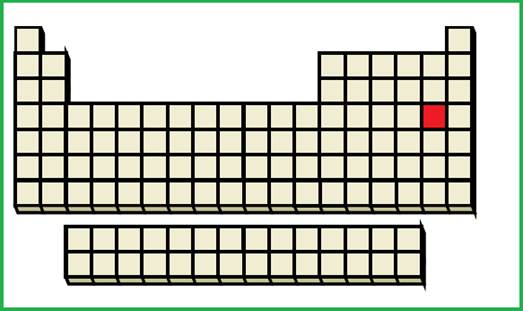
Figure 4
Want to see more full solutions like this?
Chapter 2 Solutions
General Chemistry: Atoms First
- 1. Balance the following nuclear decay processes and indicate which type of radiation is involved. a. 59 -> b. 102 251 No 247 100 Fm +arrow_forward3. The chart here shows the decay of a particular radioisotope. What is its half-life? Exponential 1st Order Decay Mass (g) 18 16 14 12 10 8 8 6 4 2 0 0 50 100 150 200 250 300 Time (min)arrow_forward3. The amount of a radioactive element decreases from 2.4 g to 0.30 g in 12 days. What is its half-life?arrow_forward
- 2. A radioactive substance has a half-life of 3 seconds. If the initial activity is 3.8 × 108 decays/second, what will the activity be after 15 seconds?arrow_forward2. Which option here correctly represents beta decay for a Xe-133 nucleus? a. 133Xe → 133 122Cs + He b. 13 Xe + _ie -> 133 c. 133 Xe→ 1331 + +₁e 54 -> d. 133 Xe → 133 Cs+-e 54 -> ven though Custom ionalarrow_forward1. 214 Po is an α emitter. Give the name and symbol of the new element that is formed during this 84 process.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





