
(a)
Interpretation:
The electronic configuration of tungsten should be determined.
Concept Introduction:
The electrons are arranged around the nucleus of an atom in an increasing order of energy levels and this description of orbitals of atom occupied by electrons is known as electronic configuration.
Answer to Problem 2.10P
Electronic configuration of tungsten is:
Explanation of Solution
Electrons are distributed in the orbitals of the sub-shell. The specific region of space in which the movements of electrons are confined is said to be shells which are divided into sub-shells and are s-, p-, d-, and f-. Among these sub-shells, the electrons are grouped as orbitals.
The numbers of electrons that these sub-shells can hold are:
s-block - 2
p-block - 6
d-block - 10
f-block - 14
The increasing order of energy of shells, sub-shell is:
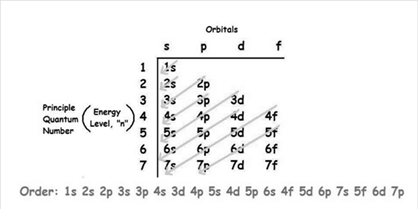
As the number of electrons for a neutral atom is equal to the atomic number of an atom so,
The atomic number of tungsten is = 74
The electronic configuration of tungsten is:
(b)
Interpretation:
The electronic configuration of cobalt should be determined.
Concept Introduction:
The electrons are arranged around the nucleus of an atom in an increasing order of energy levels and this description of orbitals of atom occupied by electrons is known as electronic configuration.
Answer to Problem 2.10P
Electronic configuration of cobalt is:
Explanation of Solution
Electrons are distributed in the orbitals of the sub-shell. The specific region of space in which the movements of electrons are confined is said to be shells which are divided into sub-shells and are s-, p-, d-, and f-. Among these sub-shells, the electrons are grouped as orbitals.
The numbers of electrons that these sub-shells can hold are:
s-block - 2
p-block - 6
d-block - 10
f-block - 14
The increasing order of energy of shells, sub-shell is:
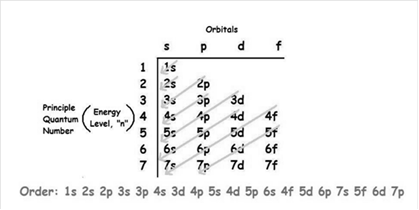
As the number of electrons for a neutral atom is equal to the atomic number of an atom so,
The atomic number of cobalt is = 27
The electronic configuration of cobalt is:
(c)
Interpretation:
The electronic configuration of zirconium should be determined.
Concept Introduction:
The electrons are arranged around the nucleus of an atom in an increasing order of energy levels and this description of orbitals of atom occupied by electrons is known as electronic configuration.
Answer to Problem 2.10P
The electronic configuration of zirconium is:
Explanation of Solution
Electrons are distributed in the orbitals of the sub-shell. The specific region of space in which the movements of electrons are confined is said to be shells which are divided into sub-shells and are s-, p-, d-, and f-. Among these sub-shells, the electrons are grouped as orbitals.
The numbers of electrons that these sub-shells can hold are:
s-block - 2
p-block - 6
d-block - 10
f-block - 14
The increasing order of energy of shells, sub-shell is:
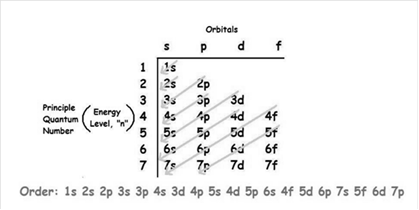
As the number of electrons for a neutral atom is equal to the atomic number of an atom so,
The atomic number of zirconium is = 40
The electronic configuration of zirconium is;
(d)
Interpretation:
The electronic configuration of uranium should be determined.
Concept Introduction:
The electrons are arranged around the nucleus of an atom in an increasing order of energy levels and this description of orbitals of atom occupied by electrons is known as electronic configuration.
Answer to Problem 2.10P
Electronic configuration of uranium is:
Explanation of Solution
Electrons are distributed in the orbitals of the sub-shell. The specific region of space in which the movements of electrons are confined is said to be shells which are divided into sub-shells and are s-, p-, d-, and f-. Among these sub-shells, the electrons are grouped as orbitals.
The numbers of electrons that these sub-shells can hold are:
s-block - 2
p-block - 6
d-block - 10
f-block - 14
The increasing order of energy of shells, sub-shell is:
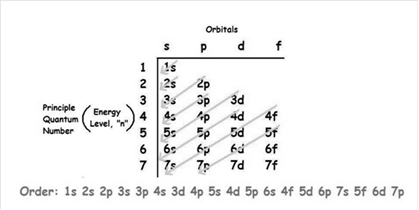
As the number of electrons for a neutral atom is equal to the atomic number of an atom so,
The electronic configuration of uranium is:
(e)
The electronic configuration of aluminum should be determined.
Concept Introduction:
The electrons are arranged around the nucleus of an atom in an increasing order of energy levels and this description of orbitals of atom occupied by electrons is known as electronic configuration.
Answer to Problem 2.10P
Electronic configuration of aluminum is:
Explanation of Solution
Electrons are distributed in the orbitals of the sub-shell. The specific region of space in which the movements of electrons are confined is said to be shells which are divided into sub-shells and are s-, p-, d-, and f-. Among these sub-shells, the electrons are grouped as orbitals.
The numbers of electrons that these sub-shells can hold are:
s-block - 2
p-block - 6
d-block - 10
f-block - 14
The increasing order of energy of shells, sub-shell is:
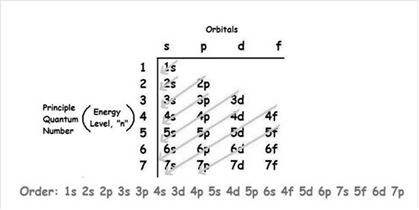
As the number of electrons for a neutral atom is equal to the atomic number of an atom so,
The atomic number of aluminum is = 13
The electronic configuration of aluminum is:
Want to see more full solutions like this?
Chapter 2 Solutions
Essentials Of Materials Science And Engineering
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





