
Fundamentals of Anatomy & Physiology Plus Mastering A&P with eText - Access Card Package (10th Edition) (New A&P Titles by Ric Martini and Judi Nath)
10th Edition
ISBN: 9780321908599
Author: Frederic H. Martini, Judi L. Nath, Edwin F. Bartholomew
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 1RQ
An oxygen atom has eight protons (a) Sketch in the arrangement of electrons around the nucleus of the oxygen atom in the following diagram (b) How many more electrons will it take to fill the outermost energy level?
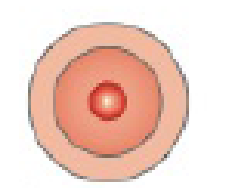
Oxygen atom
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The elements oxygen and sulfur have similar chemical properties because they both have six electrons in their outermost electron shells. Indeed, both elements form molecules with two hydrogen atoms, water (H2O) and hydrogen sulfide (H2S). Surprisingly, at room temperature, water is a liquid, yet H2S is a gas, despite sulfur being much larger and heavier than oxygen. Explain why this might be the case.
Another fossil-fuel gas used in cooking appliances is "propane", used commonly
in backyard grills and RV/camping stoves. Propane is a bigger hydrocarbon: 3
carbons single-bonded in a chain, and the remaining bonds are taken by
hydrogen. Its formula is C3H8 and its structure is shown below. KEY POINT: the
products are the same as the combustion of methane, but more is produced.
The graphic below shows that one propane molecule reacts with 5 oxygen
molecules to form
molecules of carbon dioxide.
C3Hg + 502 → 3CO,+ 4H,0
00O
000
SUOLDICIATEFIEETILNED I RLIANMr
CASIRICTIOonL
H
The “octet rule” in chemistry helps predict the types of bonds thatatoms will form. In general, an atom will be most stable if it fills itsouter shell of 8 electrons. Atoms with fewer than 4 valence electronstend to donate electrons and those with more than 4 valence electronstend to accept additional electrons; those with exactly 4 can do both.Using this rule, determine what category each of the followingelements falls into: N, S, C, P, O, H, Ca, Fe, and Mg. (You will needto work out the valence of the atoms.)
Chapter 2 Solutions
Fundamentals of Anatomy & Physiology Plus Mastering A&P with eText - Access Card Package (10th Edition) (New A&P Titles by Ric Martini and Judi Nath)
Ch. 2 - Define atom.Ch. 2 - Atoms of the same element that have different...Ch. 2 - How is it possible for two samples of hydrogen to...Ch. 2 - Prob. 4CPCh. 2 - Prob. 5CPCh. 2 - Both oxygen and neon are gases at room temperature...Ch. 2 - Prob. 7CPCh. 2 - Prob. 8CPCh. 2 - Prob. 9CPCh. 2 - Prob. 10CP
Ch. 2 - What is an enzyme?Ch. 2 - Prob. 12CPCh. 2 - Prob. 13CPCh. 2 - Explain how the chemical properties of water make...Ch. 2 - Define pH, and explain how the pH scale relates to...Ch. 2 - What is the significance of pH in physiological...Ch. 2 - Define the following terms: acid, base, and salt.Ch. 2 - Prob. 18CPCh. 2 - Prob. 19CPCh. 2 - Describe lipids.Ch. 2 - Prob. 21CPCh. 2 - Prob. 22CPCh. 2 - Prob. 23CPCh. 2 - Describe a nucleic acid.Ch. 2 - Prob. 25CPCh. 2 - Describe ATP.Ch. 2 - What molecule is produced by the phosphorylation...Ch. 2 - 28. Identify the biochemical building blocks...Ch. 2 - 29. Define metabolic turnover.
Ch. 2 - An oxygen atom has eight protons (a) Sketch in the...Ch. 2 - What is the following type of decomposition...Ch. 2 - The subatomic particle with the least mass (a)...Ch. 2 - Isotopes of an element differ from each other in...Ch. 2 - The number and arrangement of electrons in an...Ch. 2 - All organic compounds in the human body contain...Ch. 2 - A substance containing atoms of different elements...Ch. 2 - All the chemical reactions that occur in the human...Ch. 2 - Which of the following chemical equations...Ch. 2 - Prob. 10RQCh. 2 - A pH of 7.8 in the human body typifies a condition...Ch. 2 - A(n) _____ is a solute that dissociates to release...Ch. 2 - Special catalytic molecules called _____ speed up...Ch. 2 - Which of the following is not a function of a...Ch. 2 - Complementary base pairing in DNA includes the...Ch. 2 - What are the three subatomic panicles in atoms?Ch. 2 - Prob. 17RQCh. 2 - Prob. 18RQCh. 2 - List seven major functions performed by proteins.Ch. 2 - (a) What three basic components make up a...Ch. 2 - What three components are required to create the...Ch. 2 - If a polypeptide contains 10 peptide bonds, how...Ch. 2 - Prob. 23RQCh. 2 - Prob. 24RQCh. 2 - What is a salt? How does a salt differ from an...Ch. 2 - Prob. 26RQCh. 2 - In an exergonic reaction, (a) large molecules are...Ch. 2 - Prob. 28RQCh. 2 - Prob. 29RQCh. 2 - An atom of the element calcium has 20 protons and...Ch. 2 - A certain reaction pathway consists of four steps....Ch. 2 - Prob. 32RQCh. 2 - Prob. 1CCCh. 2 - Prob. 2CC
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Kevin was experiencing heartburn prior to his heart attack, for which he was taking an antacid containing calcium carbonate (CaCO3). Which of the following statements is true concerning the electron shells associated with CaCO3?arrow_forwardExamine the chemical equation below, and label each reactant and product. (which is a reactant and product) H2O + CO2 ⇋ H2CO3 ⇋ HCO3- + H+ If you are blowing CO2 from your lung into a water solution, will it make the solution more acidic or basic?arrow_forwardIn living cells, glucose, a six-carbon molecule, is converted into two three-carbon molecules. What type of chemical reaction is this?arrow_forward
- Which molecule contains more energy: carbon dioxide (CO2)or ethanol (CH3CH2-OH)? Explainarrow_forward. Give the name of and symbol for an element with this number of valence electrons.a) 2b) 6c) 8arrow_forwardUsing collision theory, indicate which of the following statements regarding physical nature of the reactants is true: a) physical nature of reactants The physical nature of reactants does not influence the rate of reaction because reactions happen only on the molecular level. Solids, liquids or gases all influence the rate of reaction the same because they are all in the physical state. Solid state reactants react faster if the particle sizes are larger because reactions occur at the boundary surface with direct contact. Gaseous-state reactions are faster than liquid-state or solid-state reactions because collisions between reactants are more frequent.arrow_forward
- How many valence electrons are in Oxygenarrow_forwardWhich of the following compounds would you expect to have the highest boiling pointand which the lowest boiling point? Explain your answer.(a) CH3OCH3 (b) CH3COOH (c) CH3CH2CH3arrow_forwardComplete the table below, using the diagram of an atom shown at right. name symbol 0 proton e Properties of subatomic particles charge (in multiples of e) 0 0 0 approximate mass (amu) (choose one) ✓ (choose one) ✓ 1.0 location on diagram (choose one) ✓ (choose one) ✓ A X A Sarrow_forward
- 1. a. What is the abbreviated name for the molecule below? (3 letters) What is the abbreviated name of this molecule if it has two phosphates? What is the abbreviated name of this molecule if it has one phosphate? From your answers above, circle the form of the molecule that has the most energy. What type of energy is this? circle all that apply: (KINETIC / POTENTIAL / CHEMICAL) Where is the energy? b. Identify the three main parts of this molecule. (write on the red brackets i-iii) c. Parts ii and ii together make (blue vertical bracket) ii. NH2 НО-Р ОН ОН ОН ОН ОН iii d. What is the specific name for this molecule? e. This molecule is also a monomer building block of which biomolecule (be specific) The general name for this type of monomer is 2. a. Put arrows by the two high energy bonds on the molecule above. Explain why these functional groups are difficult to join. (hint: these are acids. Circle the H that will be donated at cellular pH). (MORE / LESS/ the SAME amount of) energy…arrow_forwardBalance these chemical equations. (Use the lowest possible whole number coefficients.) (d) NH3 + O2 → NO2 + H2O(e) Fe(OH)2 + O2 + H2O → Fe(OH)3 (f) P2H4 → PH3 + P4arrow_forward(a) A homogeneous mixture which contains water as a solvent is called (b) Ni(CIO4)2-6H2O is hydrated whereas Ni(CIO.)e is (c) NaCl contains an bond whereas O2(g) contains a bond (d) A homogeneous mixture has a and composition (e) Temperature is an because it does not depend on the amount of substance (f) The maximum number of electrons that an orbital can have is (9) The energy of the lowest level in the H atom is (h) Arrange the following subshells in the H atom in order of increasing energy: 3s 4d 2р 4f 3d 2s 3p () Wavelength and frequency of radiation have an relationshiparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license