
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1.9, Problem 1.19P
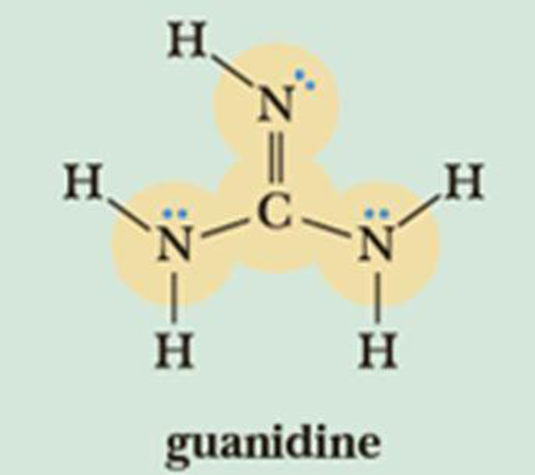
Draw three contributing structures of the following compound (called guanidine) and state the hybridization of the four highlighted atoms. In which orbitals do the three lone pairs drawn reside?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
For the indicated atoms, what is their hybridization? In what orbital do the lone pairs
reside?
For each statement, indicate whether it is true or false. (a) The greater the orbital overlap in a bond, the weaker the bond. [b] The greater the orbital overlap in a bond, the shorter the bond. [c] To create a hybrid orbital, you could use the s orbital on one atom with a p orbital on another atom. [d] Nonbonding electron pairs cannot occupy a hybrid orbital.
carbon is the master of hybridization, name 3 different compounds that showcase 3 different hybridization variants in the carbon atom. Include compond names, lewis diagram and the hybridization of the carbon atom.
For each of the following compounds draw the lewis diagram. Include VSEPR family, the VSEPR shape?
Determine the color of light emitted from an electron in a hydrogen atom that falls from n = 6 to n = 2.
Chapter 1 Solutions
Organic Chemistry
Ch. 1.1 - Prob. 1.1PCh. 1.2 - Prob. 1.2PCh. 1.2 - Judging from their relative positions in the...Ch. 1.2 - Classify each bond as nonpolar covalent or polar...Ch. 1.2 - Using the symbols and +, indicate the direction...Ch. 1.2 - Draw Lewis structures showing all valence...Ch. 1.2 - Draw Lewis structures for these ions and show...Ch. 1.3 - Draw Lewis structures and condensed structural...Ch. 1.3 - Prob. 1.9PCh. 1.3 - Prob. 1.10P
Ch. 1.3 - Prob. 1.11PCh. 1.3 - Prob. 1.12PCh. 1.4 - Predict all bond angles for these molecules. (a)...Ch. 1.5 - The geometry of carbon in diamond is tetrahedral,...Ch. 1.5 - Because of their spherical shape, C60 molecules...Ch. 1.5 - What best describes the CCC bond angles in C60? 1....Ch. 1.5 - Prob. 1.14PCh. 1.7 - Describe the bonding in these molecules in terms...Ch. 1.8 - Prob. 1.16PCh. 1.8 - Prob. 1.17PCh. 1.8 - Prob. 1.18PCh. 1.9 - Draw three contributing structures of the...Ch. 1.9 - What is the hybridization state of the circled...Ch. 1.9 - The molecule shown on the right in the example in...Ch. 1.9 - Prob. CQCh. 1.9 - The following structure is called imidazolium....Ch. 1 - Write the ground-state electron configuration for...Ch. 1 - Identify the atom that has each ground-state...Ch. 1 - Define valence shell and valence electron.Ch. 1 - How many electrons are in the valence shell of...Ch. 1 - Prob. 1.24PCh. 1 - Prob. 1.25PCh. 1 - Prob. 1.26PCh. 1 - Write Lewis structures for these compounds. Show...Ch. 1 - Write Lewis structures for these ions. Show all...Ch. 1 - Prob. 1.29PCh. 1 - Some of these structural formulas are incorrect...Ch. 1 - Following the rule that each atom of carbon,...Ch. 1 - Following are several Lewis structures showing all...Ch. 1 - Which statements are true about electronegativity?...Ch. 1 - Why does fluorine, the element in the upper right...Ch. 1 - Arrange the single covalent bonds within each set...Ch. 1 - Using the values of electronegativity given in...Ch. 1 - Prob. 1.37PCh. 1 - Use VSEPR to predict bond angles about each...Ch. 1 - Use VSEPR to predict bond angles about each atom...Ch. 1 - Use VSEPR to predict the geometry of these ions....Ch. 1 - Prob. 1.41PCh. 1 - Prob. 1.42PCh. 1 - What is the meaning of the term tertiary (3) when...Ch. 1 - What is the meaning of the term tertiary (3) when...Ch. 1 - Draw structural formulas for (a) The four primary...Ch. 1 - Draw structural formulas for the three tertiary...Ch. 1 - Prob. 1.47PCh. 1 - Identify the functional groups in each compound.Ch. 1 - Draw a three-dimensional representation for each...Ch. 1 - Tetrafluoroethylene, C2F4, is the starting...Ch. 1 - Which statements are true about resonance...Ch. 1 - Prob. 1.52PCh. 1 - Prob. 1.53PCh. 1 - Prob. 1.54PCh. 1 - Are the structures in each set valid contributing...Ch. 1 - State the orbital hybridization of each...Ch. 1 - Describe each highlighted bond in terms of the...Ch. 1 - Following is a structural formula of the...Ch. 1 - Draw a Lewis structure for methyl isocyanate,...Ch. 1 - What is the hybridization of the highlighted atoms...Ch. 1 - Using cartoon representations, draw a molecular...Ch. 1 - In what kind of orbitals do the lone-pair...Ch. 1 - Draw the delocalized molecular orbitals for the...Ch. 1 - Prob. 1.64APCh. 1 - Each compound contains both ions and covalent...Ch. 1 - Predict whether the carbon-metal bond in these...Ch. 1 - Prob. 1.67APCh. 1 - Phosphorus is immediately under nitrogen in the...Ch. 1 - Draw a Lewis structure for the azide ion, N3. (The...Ch. 1 - Cyanic acid, HOCN, and isocyanic acid, HNCO,...Ch. 1 - In Chapter 6, we study a group of organic cations...Ch. 1 - Many reactions involve a change in hybridization...Ch. 1 - Following is a structural formula of benzene,...Ch. 1 - Following are three contributing structures for...Ch. 1 - (a) Draw a Lewis structure for the ozone molecule,...Ch. 1 - The following two compounds are isomers; that is,...Ch. 1 - In future chapters, we will encounter...Ch. 1 - Prob. 1.78AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Aspartame is a compound that is 200 times sweeter than sugar and is used extensively (under the trade name NutraSweet) in diet soft drinks. The skeleton structure of the atoms in aspartame is (a) Complete the Lewis structure and give the number of and bonds in aspartame. (b) What is the hybridization about each carbon atom that forms a double bond with an oxygen atom? (c) What is the hybridization about each nitrogen atom?arrow_forward2) a) Consider the following molecule . Given what you have learned about hybridization theory, draw an image or images explaining the bonding situation in this molecule. I want you to draw out all of the orbitals, hybrid orbitals and how they overlap to form the bonds in the molecule. Indicate the % s or p character in the given atomic and hybrid orbitals. Which C-C bond or bonds are the longest? In a paragraph or so explain the image or images you just drew. b) Lastly, consider the molecule below. Indicate the Molecular formula, the molar mass, label the hybridization of each atom except for hydrogen, indicate any chiral centers with a *, which bond or bonds are the shortest, identify by name of each functional group with an arrow pointing to the group.arrow_forwardGive detailed Solution with explanation needed..don't give Handwritten answerarrow_forward
- I have a question regarding hybrid vs atomic orbitals: 1) What would be the hybridizations of C and O in the molecule H2CO? It asks to draw an electron orbital diagram (with arrows) of all the electrons in C before and after hybridization. 2) Draw a diagram of the H2CO molecule showing the overlap of orbitals between the C and O atoms that make up the sigma (σ) and pi (π) bonds. Which orbitals make up the σ bond? Which ones form the π bond?arrow_forwardGive detailed Solution with explanation neededarrow_forwardGive detailed Solution with explanation needed. Don't give Handwritten answerarrow_forward
- 2. Hybridized Orbital Diagram • Determine the hybridization of the indicated atom • Sketch the hybridization diagram for the hybridized atom •Label the orbitals according to their structural role (empty p-orbital; lone pair; a-bond; z-bond) E Fatom hybridization E H H CB-H B atom hybridizationarrow_forwardOctocrylene is an ingredient found in topical sunscreens. It is a water-resistant molecule that helps protect skin against harmful UVA and UVB radiation. Octocrylene Please answer the following questions: (a) What is the hybridisation of each nonhydrogen atom? (b) Are there two unique configurations possible about the C=C double bond? Please explain your answer. (c) Which of the two C-C bonds indicated by the arrows would you expect to be shorte.? Please explain your answer.arrow_forwarda) Which atom is most likely to be protonated on the structure below? Explain. b) Which atomic/hybrid orbitals are combining to form the indicated bonds? H₂N OHarrow_forward
- Give detailed Solution with explanation needed (no need Handwrittenarrow_forwardAn organic chemist synthesizes the molecule below:(a) Which of the orientations of hybrid orbitals shown below a represent in the molecule? (b) Are there any present that are not shown below? If so, what are they? (c) How many of each type of hybrid orbital are present?arrow_forwardPredict the hybridization and the bond angles around the indicated atoms. H b NEC-0-Ċ-H methyl cyanate Atom a Atom b Atom c Hybridization Bond anglearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY