
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
4th Edition
ISBN: 9780134110684
Author: Randall D. Knight (Professor Emeritus)
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 72EAP
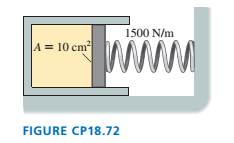
The cylinder in FIGURE CP18.72 has a moveable piston attached
to a spring. The cylinder's cross-section area is 10 cm2, it contains
0.0040 mol of gas, and the spring constant is 1500 N/m. At 20oC
the spring is neither compressed nor stretched. How far is the
spring compressed if the gas temperature is raised to 100oC?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
need help part e
Critical damping is the case where the mass never actually crosses over equilibrium position, but reaches equilibrium as fast as possible. Experiment with changing c to find the critical damping constant. Use the same initial conditions as in the last problem. Zoom in a bit to make sure you don't allow any oscillations to take place - even small ones.
NASA's KC-135 Reduced Gravity Research aircraft, affectionately known as the "Vomit Comet," is used in training astronauts and testing equipment for microgravity environments. During a typical mission, the aircraft makes approximately 30 to 40 parabolic arcs. During each arc, the aircraft and objects inside it are in free-fall, and
passengers float freely in apparent "weightlessness."
The figure below shows the altitude of the aircraft during a typical mission. It climbs from 24,000 ft to 30,850 ft, where it begins a parabolic arc with a velocity of 155 m/s at 45.0° nose-high and exits with velocity 155 m/s at 45.0° nose-low.
31 000
45° nose high
45° nose low
24 000
Zero g
65
Maneuver time (s)
(a) What is the aircraft's speed (in m/s) at the top of the parabolic arc?
110.0
m/s
(b) What is the aircraft's altitude (in ft) at the top of the parabolic arc?
2.04e+04
What is the initial height at the start of the parabolic arc? What is the initial velocity at this point? What is the final…
Chapter 18 Solutions
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Ch. 18 - Prob. 1CQCh. 18 - Prob. 2CQCh. 18 - Prob. 3CQCh. 18 - Prob. 4CQCh. 18 - Prob. 5CQCh. 18 - Prob. 6CQCh. 18 - Prob. 7CQCh. 18 - Prob. 8CQCh. 18 - Prob. 9CQCh. 18 - A gas undergoes the process shown in FIGURE...
Ch. 18 - Prob. 11CQCh. 18 - Prob. 12CQCh. 18 - Prob. 1EAPCh. 18 - Prob. 2EAPCh. 18 - What is the diameter of a copper sphere that has...Ch. 18 - Prob. 4EAPCh. 18 - Prob. 5EAPCh. 18 - How many atoms are in a 2.0 cm × 2.0 cm × 2.0 cm...Ch. 18 - Prob. 7EAPCh. 18 - An element in its solid phase has mass density...Ch. 18 - .0 mol of gold is shaped into a sphere. What is...Ch. 18 - What volume of aluminum has the same number of...Ch. 18 - Prob. 11EAPCh. 18 - Prob. 12EAPCh. 18 - Prob. 13EAPCh. 18 - A concrete bridge is built of 325-cm-long concrete...Ch. 18 - A surveyor has a steel measuring tape that is...Ch. 18 - Two students each build a piece of scientific...Ch. 18 - Prob. 17EAPCh. 18 -
18. What is the temperature in °F and the...Ch. 18 - Prob. 19EAPCh. 18 - .0 mol of gas at a temperature of -120°C fills a...Ch. 18 - Prob. 21EAPCh. 18 - Prob. 22EAPCh. 18 - Prob. 23EAPCh. 18 - Prob. 24EAPCh. 18 - Prob. 25EAPCh. 18 - Prob. 26EAPCh. 18 - Prob. 27EAPCh. 18 - Prob. 28EAPCh. 18 - A rigid, hollow sphere is submerged in boiling...Ch. 18 -
30. A rigid container holds hydrogen gas at a...Ch. 18 - Prob. 31EAPCh. 18 - Prob. 32EAPCh. 18 - Prob. 33EAPCh. 18 - Prob. 34EAPCh. 18 - Prob. 35EAPCh. 18 - Prob. 36EAPCh. 18 - Prob. 37EAPCh. 18 - .0050 mol of gas undergoes the process 1 2 3...Ch. 18 - Prob. 39EAPCh. 18 - Prob. 40EAPCh. 18 - Prob. 41EAPCh. 18 - Prob. 42EAPCh. 18 - Prob. 43EAPCh. 18 - A 15°C, 2.0-cm-diameter aluminum bar just barely...Ch. 18 - Prob. 45EAPCh. 18 - Prob. 46EAPCh. 18 - Prob. 47EAPCh. 18 - Prob. 48EAPCh. 18 - Prob. 49EAPCh. 18 - The 3.0-m-long pipe in FIGURE P18.50 is closed at...Ch. 18 - Prob. 51EAPCh. 18 - An electric generating plant boils water to...Ch. 18 - Prob. 53EAPCh. 18 - The air temperature and pressure in a laboratory...Ch. 18 - Prob. 55EAPCh. 18 - The mercury manometer shown in FIGURE P18.56 is...Ch. 18 - Prob. 57EAPCh. 18 - The 50 kg circular piston shown in FIGURE P18.58...Ch. 18 - Prob. 59EAPCh. 18 - .0 g of helium gas follows the process 1? 2 ?3...Ch. 18 - Prob. 61EAPCh. 18 - 62. FIGURE P18.62 shows two different processes...Ch. 18 - Prob. 63EAPCh. 18 - Prob. 64EAPCh. 18 - Prob. 65EAPCh. 18 - Prob. 66EAPCh. 18 - Prob. 67EAPCh. 18 - Prob. 68EAPCh. 18 - Prob. 69EAPCh. 18 - Prob. 70EAPCh. 18 - Prob. 71EAPCh. 18 - The cylinder in FIGURE CP18.72 has a moveable...Ch. 18 - Containers A and B in FIGURE CP18.73 hold the same...Ch. 18 - Prob. 74EAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 12. What could we conclude if a system has a phase trajectory that sweeps out larger and larger area as time goes by?arrow_forwardneed help part darrow_forwardA cab driver heads south with a steady speed of v₁ = 20.0 m/s for t₁ = 3.00 min, then makes a right turn and travels at v₂ = 25.0 m/s for t₂ = 2.80 min, and then drives northwest at v3 = 30.0 m/s for t3 = 1.00 min. For this 6.80-min trip, calculate the following. Assume +x is in the eastward direction. (a) total vector displacement (Enter the magnitude in m and the direction in degrees south of west.) magnitude direction For each straight-line movement, model the car as a particle under constant velocity, and draw a diagram of the displacements, labeling the distances and angles. Let the starting point be the origin of your coordinate system. Use the relationship speed = distance/time to find the distances traveled during each segment. Write the displacement vector, and calculate its magnitude and direction. Don't forget to convert min to s! m Model the car as a particle under constant velocity, and draw a diagram of the displacements, labeling the distances and angles. Let the…arrow_forward
- î A proton is projected in the positive x direction into a region of uniform electric field E = (-5.50 x 105) i N/C at t = 0. The proton travels 7.20 cm as it comes to rest. (a) Determine the acceleration of the proton. magnitude 5.27e13 direction -X m/s² (b) Determine the initial speed of the proton. 8.71e-6 magnitude The electric field is constant, so the force is constant, which means the acceleration will be constant. m/s direction +X (c) Determine the time interval over which the proton comes to rest. 1.65e-7 Review you equations for constant accelerated motion. sarrow_forwardThree charged particles are at the corners of an equilateral triangle as shown in the figure below. (Let q = 2.00 μC, and L = 0.750 m.) y 7.00 με 60.0° L 9 -4.00 μC x (a) Calculate the electric field at the position of charge q due to the 7.00-μC and -4.00-μC charges. 112 Once you calculate the magnitude of the field contribution from each charge you need to add these as vectors. KN/CI + 64 × Think carefully about the direction of the field due to the 7.00-μC charge. KN/Cĵ (b) Use your answer to part (a) to determine the force on charge q. 240.0 If you know the electric field at a particular point, how do you find the force that acts on a charge at that point? mN Î + 194.0 × If you know the electric field at a particular point, how do you find the force that acts on a charge at that point? mNarrow_forwardIn the Donkey Kong Country video games you often get around by shooting yourself out of barrel cannons. Donkey Kong wants to launch out of one barrel and land in a different one that is a distance in x of 9.28 m away. To do so he launches himself at a velocity of 22.6 m/s at an angle of 30.0°. At what height does the 2nd barrel need to be for Donkey Kong to land in it? (measure from the height of barrel 1, aka y0=0)arrow_forward
- For which value of θ is the range of a projectile fired from ground level a maximum? 90° above the horizontal 45° above the horizontal 55° above the horizontal 30° above the horizontal 60° above the horizontalarrow_forwardA map from The Legend of Zelda: The Breath of the Wild shows that Zora's Domain is 7.55 km in a direction 25.0° north of east from Gerudo Town. The same map shows that the Korok Forest is 3.13 km in a direction 55.0° west of north from Zora's Domain. The figure below shows the location of these three places. Modeling Hyrule as flat, use this information to find the displacement from Gerudo Town to Korok Forest. What is the magnitude of the displacement? Find the angle of the displacement. Measure the angle in degrees north of east of Gerudo Town.arrow_forwardRace car driver is cruising down the street at a constant speed of 28.9 m/s (~65 mph; he has a “lead” foot) when the traffic light in front of him turns red. a) If the driver’s reaction time is 160 ms, how far does he and his car travel down the road from the instant he sees the light change to the instant he begins to slow down? b) If the driver’s combined reaction and movement time is 750 ms, how far do he and his car travel down the road from the instant he sees the light change to the instant he slams on her brakes and car begins to slow down? c) If the driver’s average rate of acceleration is -9.5 m/s2 as he slows down, how long does it take him to come to a stop (use information about his speed of 28.9 m/s but do NOT use his reaction and movement time in this computation)? Please answer parts a-c. Show all work. For each question draw a diagram to show the vector/s. Show all the step and provide units in the answers. Provide answer to 2 decimal places unless stated otherwise.arrow_forward
- Below you will find 100 m split times for the American and France men’s 4x100 meter free style relay race during the 2008 Beijing Summer Olympics). Answer questions a-d. a) What was the total race time for each team, in seconds? b) Which team won the race? What was the difference in the teams’ times? c) What was the average speed for each team for the whole race? (provide answer to 3 decimal places). d) Calculate the average speed for each swimmer and report the results in a table like the one above. Remember to show the calculation steps. (provide answer to 3 decimal places). PLEASE SHOW ALL WORK AND STEPS.arrow_forwardNeed complete solution Pleasearrow_forwardBelow you will find 100 m split times for the American and France men’s 4x100 meter free style relay race during the 2008 Beijing Summer Olympics). Fill out the chart below. Calculate average speed per split (m/s). Show all work.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
A Level Physics – Ideal Gas Equation; Author: Atomi;https://www.youtube.com/watch?v=k0EFrmah7h0;License: Standard YouTube License, CC-BY