
The type of bond that holds the atom together.
Answer to Problem 3STP
The correct option is (A).
Explanation of Solution
Given:
The given diagram is
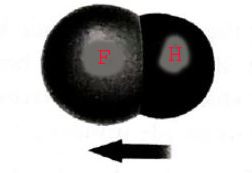
Calculation:
The figure shows the hydrogen and fluorine atom. Hydrogen and fluorine atoms are sharing their electrons and both are non-metals.
The atomic number of hydrogen is 1 and fluorine is 9.
Hydrogen requires 1 electron to complete its outermost shell. Fluorine has 7 valence electrons and fluorine requires 1 electron to get the noble gas configuration.
Therefore, both hydrogen and fluorine shares the electron with each other and form the bond. The kind of bond formed by the sharing of electrons is called a covalent bond.
Hydrogen and fluorine both are non-metal and they are sharing electrons with each other and thus form a covalent bond.
Therefore, the options (B),(C), and (D) are wrong, and option (A) is correct.
Conclusion:
Hence, the covalent bond holds the atoms shown in the above diagram.
Chapter 18 Solutions
Glencoe Physical Science 2012 Student Edition (Glencoe Science) (McGraw-Hill Education)
Additional Science Textbook Solutions
College Physics (10th Edition)
University Physics Volume 2
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Applied Physics (11th Edition)
Life in the Universe (4th Edition)
Introduction to Electrodynamics
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





