
(a)
Interpretation:
The given amine needs to be labeled as 10, 20 or 30.
Concept introduction:
Answer to Problem 18.82P
A tertiary (3o) amine.
Explanation of Solution
Amines are derivatives which are derived from ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. They can be called as alkylamines and arylamines.
Whether an amine is primary (1o), secondary (2o) or tertiary (3o) depends on the number of hydrogen atoms replaced by an alkyl or aryl group in ammonia. The amine is a primary amine if one hydrogen atom is replaced, it is a secondary amine if 2 hydrogen atoms are replaced and if three hydrogen atoms are replaced it is known as a tertiary amine.
The given compound is as follows:
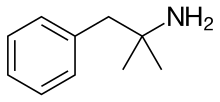
Here, only one hydrogen atom is replaced and therefore this amine called as a primary (1o) amine.
(b)
Interpretation:
The molecular shape around each atom of phentermine needs to be determined.
Concept introduction:
Molecular geometry is named as molecular shape and it is the three-dimensional arrangement of atoms within a molecule of the environment. Atoms arrange in space with an exact shape to minimize the repulsion. This can be determined using VSEPR theory.
Answer to Problem 18.82P
Carbons of benzene molecule;trigonal planar.
Carbon of −CH2 / -C(CH3)2NH2 CH2-/-CH3;tetrahedral.
Nitrogen of NH2;trigonal pyramidal.
Explanation of Solution
VSEPR or Valence Shell Electron Pair Repulsion theory is a model used to determine the geometry of molecules considering minimum electrostatic repulsion between the valence electrons of central atom in the molecule.
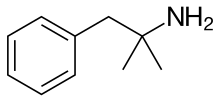
Carbons of benzene molecule;
Number of bonds= 3
Number of lone pairs = 0
Therefore, the shape is trigonal planar.
Carbon of −CH2/ -C(CH3)2NH2CH2-/-CH3;
Number of bonds= 4
Number of lone pairs = 0
Therefore, the shape is tetrahedral.
Nitrogen of NH2;
Number of bonds= 3
Number of lone pairs = 1
Therefore, the shape is trigonal pyramidal.
(c)
Interpretation:
The constitutional isomer containing a primary amine needs to be determined.
Concept introduction:
Constitutional isomers are called as compounds that have the unique molecular formula and different structural connectivity. To determine whether two molecules are constitutional isomer, the number of each atom needs to be counted in both molecules and see how the atoms are arranged.
Answer to Problem 18.82P
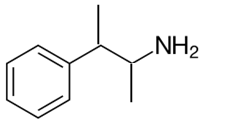
Explanation of Solution
Whether an amine is primary (1o), secondary (2o) or tertiary (3o) depends on the number of hydrogen atoms replaced by an alkyl or aryl group in ammonia. The amine is a primary amine if one hydrogen atom is replaced, it is a secondary amine if 2 hydrogen atoms are replaced and if three hydrogen atoms are replaced it is known as a tertiary amine.

Here all one hydrogen atom is replaced and therefore this amine called a primary amine (1o).
(d)
Interpretation:
The constitutional isomer that contains a secondary amine needs to be determined.
Concept introduction:
Constitutional isomers are called as compounds that have the unique molecular formula and different structural connectivity. To determine whether two molecules are constitutional isomer, the number of each atom needs to be counted in both molecules and see how the atoms are arranged.
Answer to Problem 18.82P
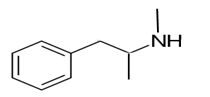
Explanation of Solution
Whether an amine is primary (1o), secondary (2o) or tertiary (3o) depends on the number of hydrogen atoms replaced by an alkyl or aryl group in ammonia. The amine is a primary amine if one hydrogen atom is replaced, it is a secondary amine if 2 hydrogen atoms are replaced and if three hydrogen atoms are replaced it is known as a tertiary amine.

Here all 3 hydrogen atoms are replaced and therefore this amine called as a secondary amine (2o).
(e)
Interpretation:
The structure of phentermine hydrobromide molecule needs to be drawn.
Concept introduction:
Phentermine hydrobromide can stimulate the central nervous system and increases the blood pressure and heart rate which decreases the appetite. It is taken along with diet and exercise in order to reduce obesity.
Answer to Problem 18.82P
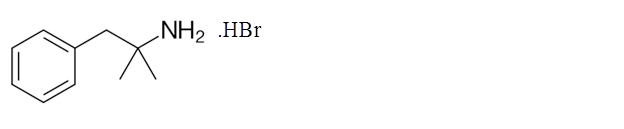
Explanation of Solution
Phentermine hydrobromide can stimulate the central nervous system and increases the blood pressure and heart rate which decreases the appetite. It is taken along with the diet and exercise in order to reduce the obesity.
Its molecular formula is C17H22BrN. It has the following structure.
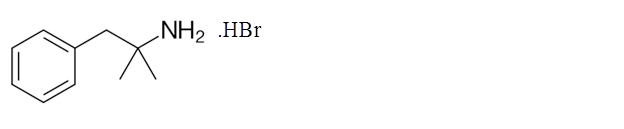
(f)
Interpretation:
The products formed if phentermine is treated with benzoic acid needs to be determined.
Concept introduction:
Amines are derivatives which are derived from ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. They can be called as alkylamines and arylamines.
Answer to Problem 18.82P
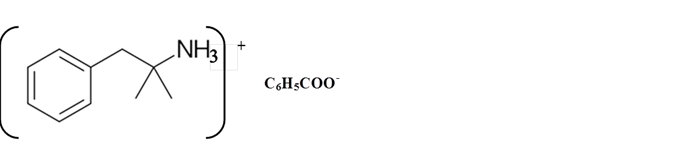
Explanation of Solution
Amines are derivatives which are derived from ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. They can be called as alkylamines and arylamines.
Whether an amine is primary (1o), secondary (2o) or tertiary (3o) depends on the number of hydrogen atoms replaced by an alkyl or aryl group in ammonia. The amine is a primary amine if one hydrogen atom is replaced, it is a secondary amine if 2 hydrogen atoms are replaced and if three hydrogen atoms are replaced it is known as a tertiary amine.
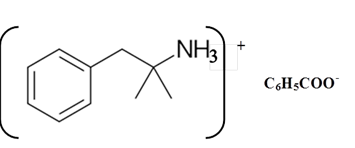
Benzphetamine is a tertiary ammine. It has basic properties and it accepts protons from acids. Acetic acid is donated a proton to this amine and formed below the salt.
Want to see more full solutions like this?
Chapter 18 Solutions
ALEKS 360 ACCESS CARD F/GEN. ORG.CHEM
- select two. Which statements correspond to: ESCITALOPRAM (antidepressant) a. It induces tranquility and somnolence to the patient. b. An agent that regulates re-uptake of serotonin in the brain. c. Its structure has benzonitrile, flurobenzene and a tertiary amine. d. An agent that is indicated to patients with hives and dermatitis.arrow_forwardMatch the description to one of the compounds E– H. a. a compound that contains a 1 ° amine and a 1 ° amide b. a compound that contains a 1 ° amine and a 2 ° amide c. a compound that contains a 2 ° amine and a 3 ° amide d. a compound that contains a 3 ° amine and a 3 ° amidearrow_forward1. What are the three kinds of alkaloids? Describe each. 2. Classify the following alkaloid according to ring system, draw the structure, then give the natural occurrence. a. betanidine b. peganine C. cocaine d. ergothioneinearrow_forward
- Reactions of aldehydes and ketones with amines and amine derivatives a. Draw reaction with a primary amine forms an imine. Hydrazine and hydroxylamine can also be used; they form a hydrazone and an oxime, respectively. b. Draw reaction with a secondary amine forms an enamine.arrow_forwardThe hydrolysis of an amide in acidic conditions forms A. a carboxylate salt and an alcohol B. a carboxylate salt and an amine C. an alcohol and an amine salt (an ammonium ion) D. a carboxylic acid and an amine salt (an ammonium ion)arrow_forward1. Which statement best described the ability of amines to hydrogen bond? A. Primary, secondary and tertiary amines can all hydrogen bond with molecules identical to themselves. B. Primary and secondary amines can hydrogen bond with molecules identical to themselves, but tertiary amines cannot. C. Only primary amines can hydrogen bond with molecules identical to themselves. Secondary and tertiary amines cannot. D. Primary, secondary and tertiary amines cannot hydrogen bond with molecules identical to themselves, but the can hydrogen bond with water.arrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts and the amine side product. 1. NaOH, heat 2. Neutralizing work-up Select to Drawarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts and the amine side product. 1. NaOH, heat N 1 2. Neutralizing work- uparrow_forwardHow is the nitrogen of the oxazole similar or different to the two nitrogen’s that are found in the pyrazole , please explain how pyrazole has been useful on everyday life of human beingsarrow_forward
- Give an acceptable name for each amine.arrow_forwardWhat amine is formed by reduction of each amide?arrow_forward5. Ritalin is the trade name for methylphenidate, a drug used to treat attention deficit hyperactivity disorder (ADHD). OCH: H -N- Methylephenidate a. Identify the functional groups. b. Label the amines as 1", 2", or 3". c. Draw the structure of methylphenidate hydrochloride.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





