
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
5th Edition
ISBN: 9780321967466
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 18.82AQAP
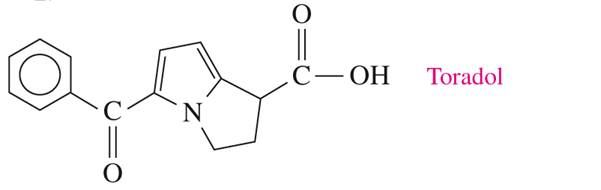
Toradol (ketorolac) is used in dentistry to relieve pain. Name the
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The table includes macrostates characterized by 4 energy levels (&) that are
equally spaced but with different degrees of occupation.
a) Calculate the energy of all the macrostates (in joules). See if they all have
the same energy and number of particles.
b) Calculate the macrostate that is most likely to exist. For this macrostate,
show that the population of the levels is consistent with the Boltzmann
distribution.
macrostate 1 macrostate 2 macrostate 3
ε/k (K) Populations
Populations
Populations
300
5
3
4
200
7
9
8
100
15
17
16
0
33
31
32
DATO: k = 1,38×10-23 J K-1
Don't used Ai solution
In an experiment, the viscosity of water was measured at different
temperatures and the table was constructed from the data obtained.
a) Calculate the activation energy of viscous flow (kJ/mol).
b) Calculate the viscosity at 30°C.
T/°C
0
20
40
60
80
η/cpoise 1,972 1,005 0,656 0,469 0,356
Chapter 18 Solutions
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
Ch. 18.1 - Prob. 18.1QAPCh. 18.1 - Prob. 18.2QAPCh. 18.1 - Prob. 18.3QAPCh. 18.1 - Prob. 18.4QAPCh. 18.1 - Prob. 18.5QAPCh. 18.1 - Prob. 18.6QAPCh. 18.1 - Prob. 18.7QAPCh. 18.1 - Prob. 18.8QAPCh. 18.1 - Prob. 18.9QAPCh. 18.1 - Prob. 18.10QAP
Ch. 18.2 - Prob. 18.11QAPCh. 18.2 - Prob. 18.12QAPCh. 18.2 - Prob. 18.13QAPCh. 18.2 - Assign the boiling point of 3 C, 48 C, or 97 C to...Ch. 18.2 - Prob. 18.15QAPCh. 18.2 - Prob. 18.16QAPCh. 18.2 - Prob. 18.17QAPCh. 18.2 - Prob. 18.18QAPCh. 18.2 - Prob. 18.19QAPCh. 18.2 - Prob. 18.20QAPCh. 18.2 - Prob. 18.21QAPCh. 18.2 - Prob. 18.22QAPCh. 18.3 - Prob. 18.23QAPCh. 18.3 - Prob. 18.24QAPCh. 18.3 - Prob. 18.25QAPCh. 18.3 - Prob. 18.26QAPCh. 18.3 - Prob. 18.27QAPCh. 18.3 - Prob. 18.28QAPCh. 18.4 - Prob. 18.29QAPCh. 18.4 - Prob. 18.30QAPCh. 18.4 - When is a neurotransmitter released into the...Ch. 18.4 - Prob. 18.32QAPCh. 18.4 - Why is it important to remove a neurotransmitter...Ch. 18.4 - Prob. 18.34QAPCh. 18.4 - Prob. 18.35QAPCh. 18.4 - Prob. 18.36QAPCh. 18.4 - Prob. 18.37QAPCh. 18.4 - Prob. 18.38QAPCh. 18.4 - Prob. 18.39QAPCh. 18.4 - Prob. 18.40QAPCh. 18.4 - Prob. 18.41QAPCh. 18.4 - Prob. 18.42QAPCh. 18.4 - Prob. 18.43QAPCh. 18.4 - What happens if there is an excess of glutamate in...Ch. 18.4 - Prob. 18.45QAPCh. 18.4 - Prob. 18.46QAPCh. 18.5 - Prob. 18.47QAPCh. 18.5 - Prob. 18.48QAPCh. 18.5 - Prob. 18.49QAPCh. 18.5 - Prob. 18.50QAPCh. 18.5 - Prob. 18.51QAPCh. 18.5 - Prob. 18.52QAPCh. 18.5 - Prob. 18.53QAPCh. 18.5 - Prob. 18.54QAPCh. 18.6 - Prob. 18.55QAPCh. 18.6 - Prob. 18.56QAPCh. 18.6 - Prob. 18.57QAPCh. 18.6 - Name the functional groups in enrofloxacin.Ch. 18.6 - Prob. 18.59QAPCh. 18.6 - Prob. 18.60QAPCh. 18 - Prob. 18.61UTCCh. 18 - 18.62 Some aspirin substitutes contain phenacetin...Ch. 18 - Neo-Synephrine is the active ingredient in some...Ch. 18 - Melatonin is a naturally occurring compound n...Ch. 18 - Prob. 18.65UTCCh. 18 - Prob. 18.66UTCCh. 18 - Prob. 18.67UTCCh. 18 - Prob. 18.68UTCCh. 18 - Give the IUPAC and common names (if any) and...Ch. 18 - Prob. 18.70AQAPCh. 18 - Prob. 18.71AQAPCh. 18 - Prob. 18.72AQAPCh. 18 - Prob. 18.73AQAPCh. 18 - Prob. 18.74AQAPCh. 18 - In each of the following pairs, indicate the...Ch. 18 - Prob. 18.76AQAPCh. 18 - Give the IUPAC name for each of the following...Ch. 18 - Give the IUPAC name for each of the following...Ch. 18 - Prob. 18.79AQAPCh. 18 - Prob. 18.80AQAPCh. 18 - Prob. 18.81AQAPCh. 18 - Toradol (ketorolac) is used in dentistry to...Ch. 18 - Prob. 18.83AQAPCh. 18 - Prob. 18.84AQAPCh. 18 - Prob. 18.85AQAPCh. 18 - What is the structural difference between...Ch. 18 - What does the abbreviation SSRI signify? (18.4)Ch. 18 - Give an example of an SSRI and its role in...Ch. 18 - 18.89 There are four amine isomers with the...Ch. 18 - Prob. 18.90CQCh. 18 - Use the Internet to look up the structural formula...Ch. 18 - Prob. 18.92CQCh. 18 - Prob. 18.93CQCh. 18 - Prob. 18.94CQCh. 18 - Prob. 35CICh. 18 - Prob. 36CICh. 18 - Prob. 37CICh. 18 - Prob. 38CICh. 18 - Prob. 39CICh. 18 - Prob. 40CI
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used Ai solutionarrow_forwardLet's see if you caught the essentials of the animation. What is the valence value of carbon? a) 4 b) 2 c) 8 d) 6arrow_forwardA laser emits a line at 632.8 nm. If the cavity is 12 cm long, how many modes oscillate in the cavity? How long does it take for the radiation to travel the entire cavity? What is the frequency difference between 2 consecutive modes?(refractive index of the medium n = 1).arrow_forward
- A laser emits a line at 632.8 nm. If the cavity is 12 cm long, how many modes oscillate in the cavity? How long does it take for the radiation to travel the entire cavity? What is the frequency difference between 2 consecutive modes?(refractive index of the medium n = 1).arrow_forwardThe number of microstates corresponding to each macrostate is given by N. The dominant macrostate or configuration of a system is the macrostate with the greatest weight W. Are both statements correct?arrow_forwardFor the single step reaction: A + B → 2C + 25 kJ If the activation energy for this reaction is 35.8 kJ, sketch an energy vs. reaction coordinate diagram for this reaction. Be sure to label the following on your diagram: each of the axes, reactant compounds and product compounds, enthalpy of reaction, activation energy of the forward reaction with the correct value, activation energy of the backwards reaction with the correct value and the transition state. In the same sketch you drew, after the addition of a homogeneous catalyst, show how it would change the graph. Label any new line "catalyst" and label any new activation energy.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY