
(a)
Interpretation:
The structure of amide formed by the reaction of propylamine
Concept Introduction:
Answer to Problem 60P
The propanoic acid
Explanation of Solution
The reaction of carboxylic acid with ammonia or amines forms amide molecules. It involves the formation of water molecule. In this reaction the amine nitrogen atom react with carbonyl carbon atom of carboxylic acid to form amide.
In the reaction of
(b)
Interpretation:
The structure of amide formed by the reaction of
Concept Introduction:
Functional groups are the groups of atoms or atoms which are bonded with parent carbon chain in the organic molecule and are responsible for the physical and chemical properties of the compound. In organic chemistry, there are different functional groups such as carboxylic acid, alcohol, ester, or amide.
Amines are the organic compounds with general chemical formula of R-NH2 or R-NH-R whereas carboxylic acids are the organic molecules with R-COOH as general chemical formula.
Answer to Problem 60P
The Dodecanoic acid
Explanation of Solution
The reaction of carboxylic acid with ammonia or amines forms amide molecules. It involves the formation of water molecule. In this reaction the amine nitrogen atom react with carbonyl carbon atom of carboxylic acid to form amide.
In the reaction of
(c)
Interpretation:
The structure of amide formed by the reaction of
Concept Introduction:
Functional groups are the groups of atoms or atoms which are bonded with parent carbon chain in the organic molecule and are responsible for the physical and chemical properties of the compound. In organic chemistry, there are different functional groups such as carboxylic acid, alcohol, ester, or amide.
Amines are the organic compounds with general chemical formula of R-NH2 or R-NH-R whereas carboxylic acids are the organic molecules with R-COOH as general chemical formula.
Answer to Problem 60P
The formic acid
Explanation of Solution
The reaction of carboxylic acid with ammonia or amines forms amide molecules. It involves the formation of water molecule. In this reaction the amine nitrogen atom react with carbonyl carbon atom of carboxylic acid to form amide.
In the reaction of
(d)
Interpretation:
The structure of amide formed by the reaction of p-methoxybenzoic acid with
Concept Introduction:
Functional groups are the groups of atoms or atoms which are bonded with parent carbon chain in the organic molecule and are responsible for the physical and chemical properties of the compound. In organic chemistry, there are different functional groups such as carboxylic acid, alcohol, ester, or amide.
Amines are the organic compounds with general chemical formula of R-NH2 or R-NH-R whereas carboxylic acids are the organic molecules with R-COOH as general chemical formula.
Answer to Problem 60P
The p-methoxybenzoic acid reacts with
Explanation of Solution
The reaction of carboxylic acid with ammonia or amines forms amide molecules. It involves the formation of water molecule. In this reaction the amine nitrogen atom react with carbonyl carbon atom of carboxylic acid to form amide.
In the reaction of
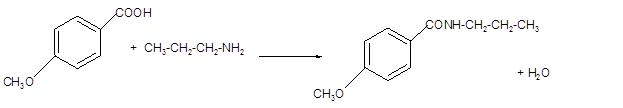
Want to see more full solutions like this?
Chapter 17 Solutions
Connect One Semester Access Card for General, Organic, & Biological Chemistry
- 50. If this molecule was heated, which carboxyl group will be readily lost as carbon dioxid. C HO2C CO2H CO2H HO2C B a. b. B C. C d.arrow_forwardWhat is the systematic IUPAC name for the given compound? CH3 CH3 CH;CHCH,CH,CH2 -Ń-CH3 a. N-methyl-4-methylhexan-1-amine b. 2,4-dimethylhexan-1-amine c. 2,2,N-trimethylpentan-1-amine d. N,N,4-trimethylpentan-1-aminearrow_forward2. What is produced when an amine reacts with water? A. A primary alcohol and ammonia B. An amide and a hydrogen (H+) ion C. An ammonium ion and a hydroxide (OH-) ion D. An amide and a hydroxide (OH-) ionarrow_forward
- amine, (2) an amide, or (3) both an amine and an amide. 17-106 Classify each of the following compounds as (1) an amine, (2) an amide, or (3) both an amine and an NH2 b. `NH a. H2N H d. с.arrow_forward19. Which of the following molecules contain an amide? H2N-CHC-OH ČH3 a. c. NH2 NH2 b. H2N H2N-CHC-N-CHC-OH d. CHз CH3arrow_forwardC. Amides 1. Amidation a. Acetic acid + ammoniaarrow_forward
- Name each amine. b. CH;-CH2-CH2-N-CH, H CH3 c. CH3-CH2-CH;-N-CH,-CH;-CH2-CH;arrow_forwardName each amine. CH;-CH,-N-CH,-CH, b. CH3-CH,-CH2-N-CH3 H CH3 CH3-CH2-CH2-N-CH2-CH;-CH2-CH3arrow_forwardWhat are the products of the reaction between acetyl chloride and diethylamine? HCl and N,N-diethylacetamide Оа. o b. HCl and diethylamine chloride ос. HCl and N-ethylacetamide d. Diethylacetamide and acidarrow_forward
- 2. What is produced when an amine reacts with a strong acid such as HCl? A. An amine and the OH- ion B. An amide and the H+ ion C. An ammonium hydroxide D. An ammonium saltarrow_forward1. Is C10H140 an aldehyde or ketone? 2. Aspartic acid belongs to: a. carboxylic acid b. acid anhydride c. acid halide d. amides e. esters 3. Which is least reactive? a. carboxylic acid b. acid anhydride c. acid halide d. amides e. estersarrow_forwardName each amine. a. CH3-CH2-N-CH2-CH, Harrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


