
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 19P
Give an IUPAC systematic or common name for each of the following compounds:
(a)
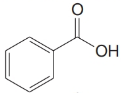
(b)
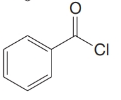
(c)
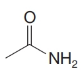
(d)
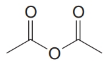
(e)
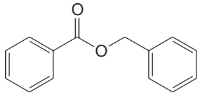
(f)
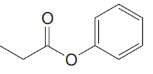
(g)
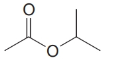
(h)
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please correct answer and don't used hand raiting
Curved arrows are used to illustrate the flow of electrons. Using the provided
starting and product structures, draw the curved electron-pushing arrows for
the following reaction or mechanistic step(s).
Be sure to account for all bond-breaking and bond-making steps.
Select to Edit Arrows
H
H
Select to Add Arrows
>
H
CFCI:
Select to Edit Arrows
H
Select to Edit Arrows
Show work with explanation needed. don't give Ai generated solution
Chapter 17 Solutions
Organic Chemistry
Ch. 17 - Practice Problem 17.1 Give an IUPAC systematic...Ch. 17 - Prob. 2PPCh. 17 - PRACTICE PROBLEM
17.3 Which acid of each pair...Ch. 17 - Practice Problem 17.3 Write structural formulas...Ch. 17 - Practice Problem 17.4
Show how each of the...Ch. 17 - Practice Problem 17.5
Show how you could prepare...Ch. 17 - Practice Problem 17.6
(a) Which of the carboxylic...Ch. 17 - Prob. 8PPCh. 17 - Prob. 9PPCh. 17 - Practice Problem 17.9
Esters can also be...
Ch. 17 - Prob. 11PPCh. 17 - Prob. 12PPCh. 17 - Practice Problem 17.12
What products would you...Ch. 17 - Practice Problem 17.13 (a) Provide the reagents...Ch. 17 - Prob. 15PPCh. 17 - Practice Problem 17.15 Using decarboxylation...Ch. 17 - Practice Problem 17.16 Diacyl peroxides, ,...Ch. 17 - Prob. 18PCh. 17 - Give an IUPAC systematic or common name for each...Ch. 17 - Prob. 20PCh. 17 - Prob. 21PCh. 17 - 17.21 What major organic product would you expect...Ch. 17 - Prob. 23PCh. 17 - Prob. 24PCh. 17 - Prob. 25PCh. 17 - Prob. 26PCh. 17 - 17.26 What products would you expect to obtain...Ch. 17 - Write structural formulas for the major organic...Ch. 17 - 17.28 Indicate reagents that would accomplish each...Ch. 17 - Write structural formulas for the major organic...Ch. 17 - Prob. 31PCh. 17 - Prob. 32PCh. 17 - Prob. 33PCh. 17 - 17.33 On heating,...Ch. 17 - Prob. 35PCh. 17 - Prob. 36PCh. 17 - 17.36 Show how pentanoic acid can be prepared from...Ch. 17 - 17.37 The active ingredient of the insect...Ch. 17 - Prob. 39PCh. 17 - Prob. 40PCh. 17 - Give stereochemical formulas for compounds AQ:...Ch. 17 - 17.41 -Glyceraldehyde can be transformed into...Ch. 17 - Prob. 43PCh. 17 - 17.44 Cantharidin is a powerful vesicant that can...Ch. 17 - Prob. 45PCh. 17 - 17.44 Given here are the NMR spectra and carbonyl...Ch. 17 - Compound X (C7H12O4) is insoluble in aqueous...Ch. 17 - 17.45 Compound Y dissolves slowly when warmed...Ch. 17 - Prob. 49PCh. 17 - Prob. 50PCh. 17 - 17.52 Starting with 1-naphthol, suggest an...Ch. 17 - Suggest a synthesis of ibuprofen (Section 5.11)...Ch. 17 - Prob. 53PCh. 17 - Prob. 54PCh. 17 - Prob. 1LGPCh. 17 - Prob. 2LGPCh. 17 - Prob. 3LGPCh. 17 - Prob. 4LGPCh. 17 - Prob. 1QCh. 17 - 17.2 Which of the following would yield...Ch. 17 - 17.3 Which reagent would serve as the basis for a...Ch. 17 - Prob. 4QCh. 17 - Complete the following synthesis.Ch. 17 - 17.6 Which of these acids would undergo...
Additional Science Textbook Solutions
Find more solutions based on key concepts
9. A 10-cm-long thin glass rod uniformly charged to +10nC and a thin plastic rod uniformly charged to nC are ...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
A wild-type fruit fly (heterozygous for gray body color and normal wings) is mated with a black fly with vestig...
Campbell Biology (11th Edition)
10.1 Indicate whether each of the following statements is characteristic of an acid, a base, or
both:
has a so...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Johnny was vigorously exercising the only joints in the skull that are freely movable. What would you guess he ...
Anatomy & Physiology (6th Edition)
37. Galactosemia is an autosomal recessive disorder caused by the inability to metabolize galactose, a componen...
Genetic Analysis: An Integrated Approach (3rd Edition)
What are the three main parts of a typical vertebra?
Principles of Anatomy and Physiology
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY