
(a)
Interpretation:
Structure of amine or quaternary ammonium salt that is obtained when dimethylamine and propyl bromide is reacted in presence of strong base has to be drawn.
Concept Introduction:
Alkylation reaction is a reaction in which the transfer of alkyl group from one molecule to another molecule takes place. While considering
(a)
Answer to Problem 17.70EP
The structure of product obtained is,
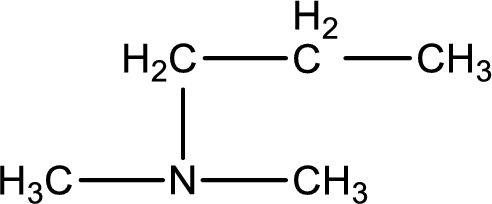
Explanation of Solution
Given reactants are dimethylamine and propyl bromide. In this, the amine given is a secondary amine. When secondary amine is treated with an alkyl halide, the product obtained is a tertiary amine. The complete reaction and the structure of the product formed is given as,
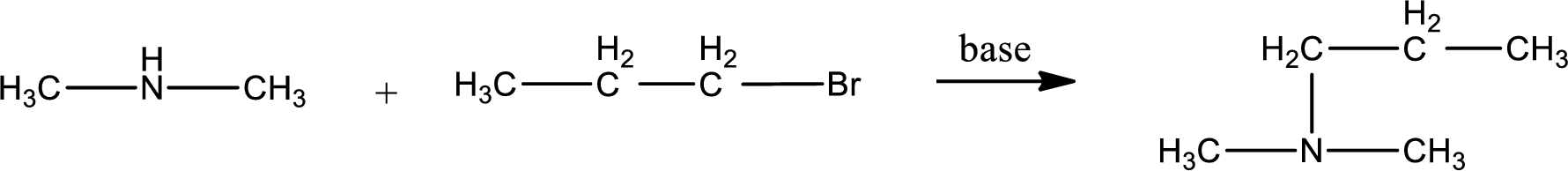
Structure of the product formed is drawn.
(b)
Interpretation:
Structure of amine or quaternary ammonium salt that is obtained when diethylmethylamine and isopropyl chloride is reacted in presence of strong base has to be drawn.
Concept Introduction:
Alkylation reaction is a reaction in which the transfer of alkyl group from one molecule to another molecule takes place. While considering amines, the alkylating agent that is used is alkyl halides. Alkylation is done under basic conditions. The general equations for amines alkylation process is,
(b)
Answer to Problem 17.70EP
The structure of product obtained is,
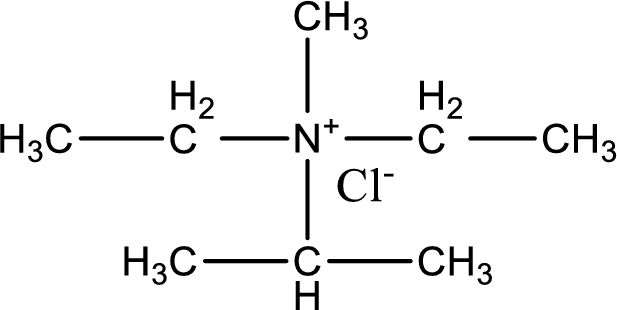
Explanation of Solution
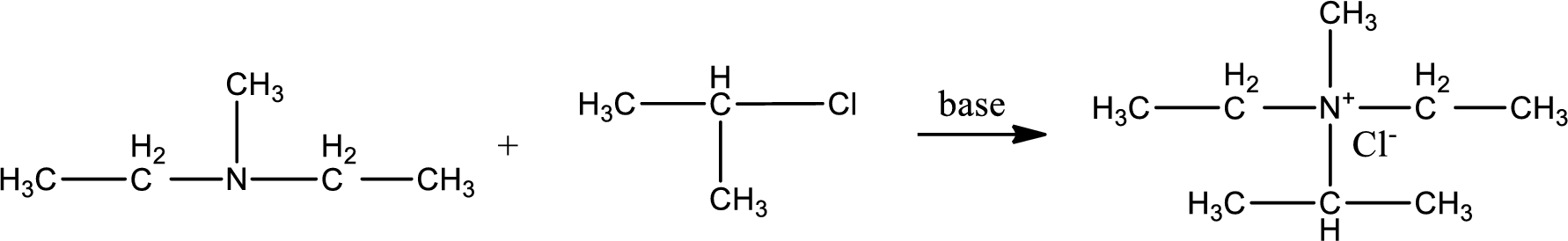
Given reactants are diethylmethylamine and isopropyl chloride. In this, the amine given is a tertiary amine. When tertiary amine is treated with an alkyl halide, the product obtained is a quaternary ammonium salt. The complete reaction and the structure of the product formed is given as,
Structure of the product formed is drawn.
(c)
Interpretation:
Structure of amine or quaternary ammonium salt that is obtained when methylpropylamine and ethyl chloride is reacted in presence of strong base has to be drawn.
Concept Introduction:
Alkylation reaction is a reaction in which the transfer of alkyl group from one molecule to another molecule takes place. While considering amines, the alkylating agent that is used is alkyl halides. Alkylation is done under basic conditions. The general equations for amines alkylation process is,
(c)
Answer to Problem 17.70EP
The structure of product obtained is,
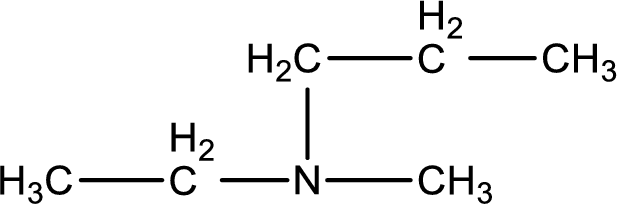
Explanation of Solution
Given reactants are methylpropylamine and ethyl chloride. In this, the amine given is a secondary amine. When secondary amine is treated with an alkyl halide, the product obtained is a tertiary amine. The complete reaction and the structure of the product formed is given as,
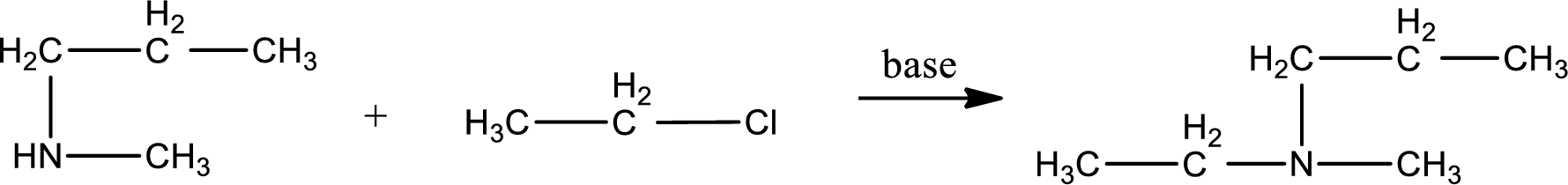
Structure of the product formed is drawn.
(d)
Interpretation:
Structure of amine or quaternary ammonium salt that is obtained when tripropylamine and propyl chloride is reacted in presence of strong base has to be drawn.
Concept Introduction:
Alkylation reaction is a reaction in which the transfer of alkyl group from one molecule to another molecule takes place. While considering amines, the alkylating agent that is used is alkyl halides. Alkylation is done under basic conditions. The general equations for amines alkylation process is,
(d)
Answer to Problem 17.70EP
The structure of product obtained is,
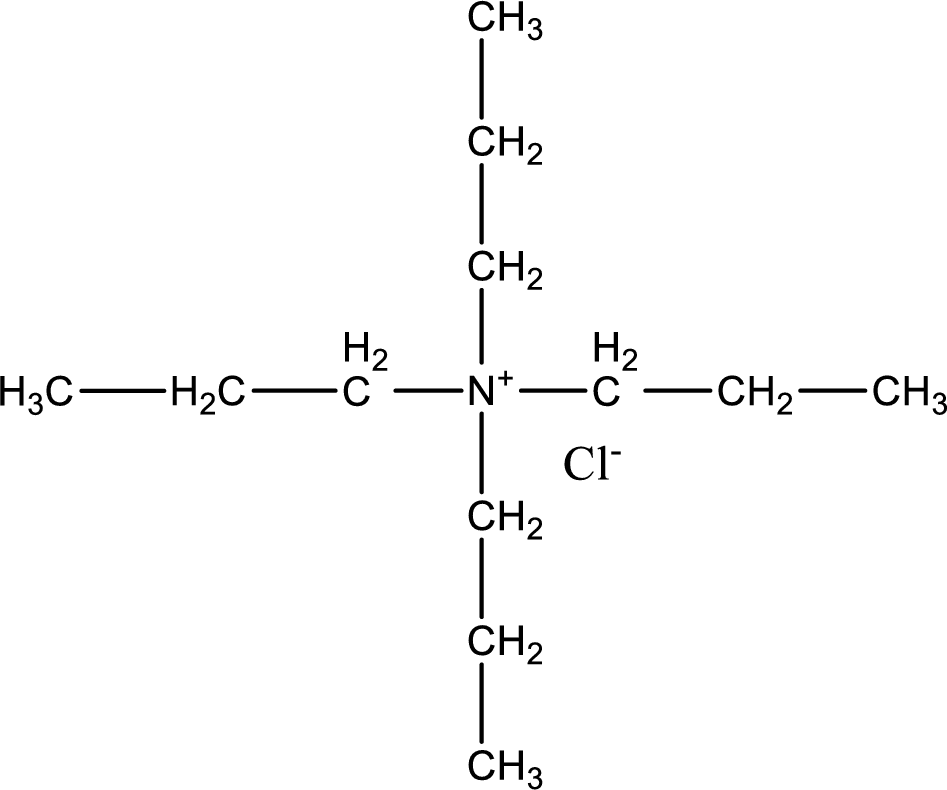
Explanation of Solution
Given reactants are tripropylamine and propyl chloride. In this, the amine given is a tertiary amine. When tertiary amine is treated with an alkyl halide, the product obtained is a quaternary ammonium salt. The complete reaction and the structure of the product formed is given as,
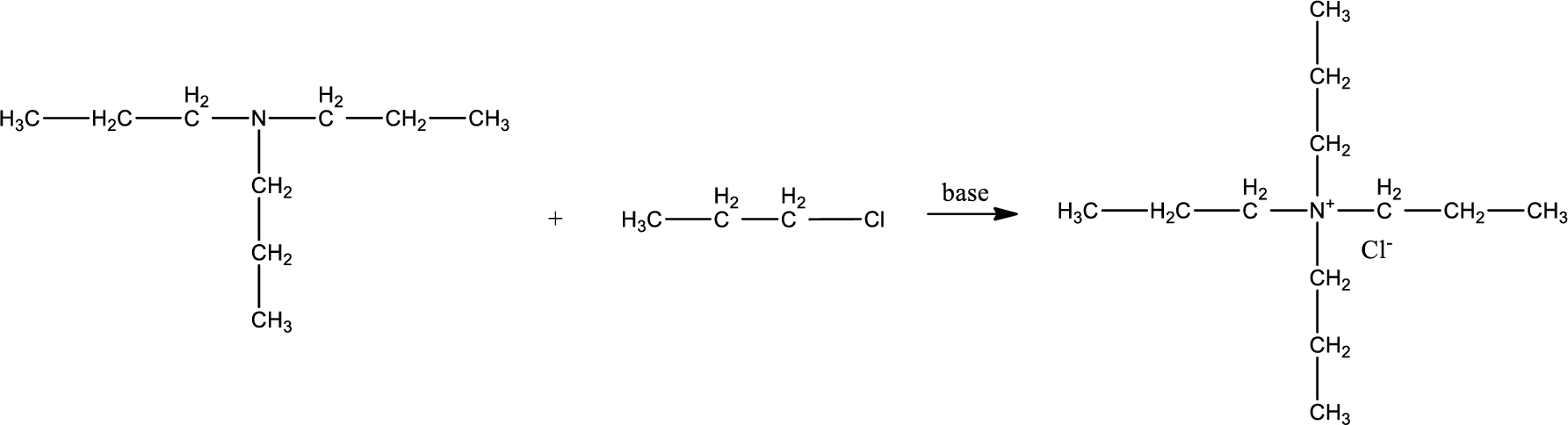
Structure of the product formed is drawn.
Want to see more full solutions like this?
Chapter 17 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
- this is an organic chemistry question please answer accordindly!! please post the solution draw the figures on a paper please hand drawn and post, please answer EACH part till the end and dont just provide wordy explanations, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forwardA mixture of 0.412 M C12, 0.544 M F2, and 0.843 M CIF is enclosed in a vessel and heated to 2500 K. C12(g) + F2(g )2CIF(g) Kc = 20.0 at 2500 K Calculate the equilibrium concentration of each gas at 2500 K. [C12] = M [F2] = M [ CIF] =arrow_forwardShow reaction mechanism with explanation. don't give Ai generated solutionarrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





