
Concept explainers
(a)
Interpretation:
Structure of nitrogen-containing compound that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between
(a)
Answer to Problem 17.136EP
Nitrogen-containing compound that is required was,
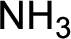
Explanation of Solution
Given structure of compound is,
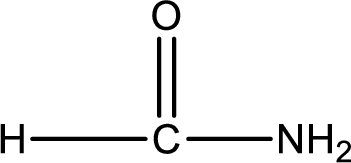
As the nitrogen atom present in the above amide has two hydrogen atoms bonded to it, the amide is a primary amide. Primary amide is produced by the reaction of ammonia with carboxylic acid. The nitrogen-containing compound can be identified as shown below,
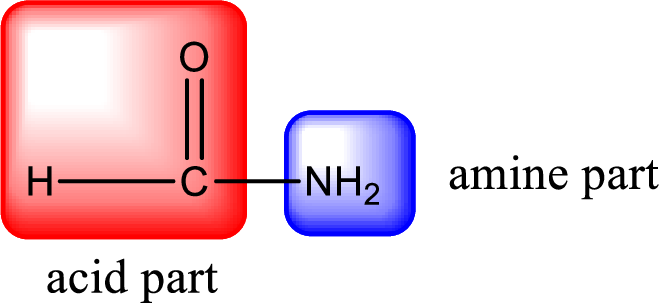
Hydrogen atom has to be added to the amine part and
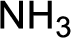
Structure of nitrogen-containing compound that is required to produce the given compound is drawn.
(b)
Interpretation:
Structure of nitrogen-containing compound that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(b)
Answer to Problem 17.136EP
Nitrogen-containing compound that is required was,
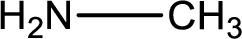
Explanation of Solution
Given structure of compound is,
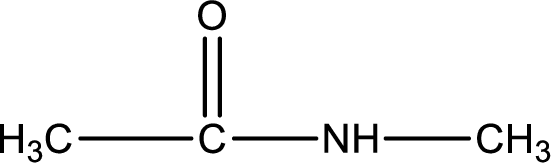
As the nitrogen atom present in the above amide has two hydrogen atoms bonded to it, the amide is a primary amide. Primary amide is produced by the reaction of ammonia with carboxylic acid. The nitrogen-containing compound can be identified as shown below,
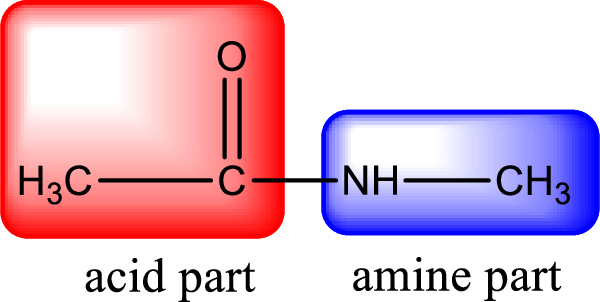
Hydrogen atom has to be added to the amine part and
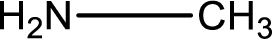
Structure of nitrogen-containing compound that is required to produce the given compound is drawn.
(c)
Interpretation:
Structure of nitrogen-containing compound that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(c)
Answer to Problem 17.136EP
Nitrogen-containing compound that is required was,
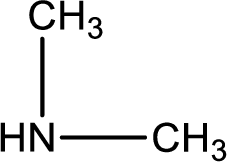
Explanation of Solution
Given structure of compound is,
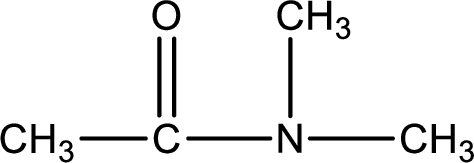
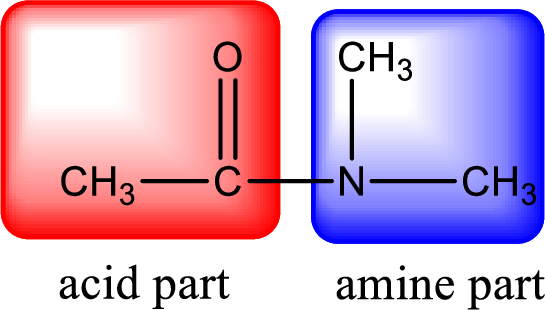
As the nitrogen atom present in the above amide has two hydrogen atoms bonded to it, the amide is a primary amide. Primary amide is produced by the reaction of ammonia with carboxylic acid. The nitrogen-containing compound can be identified as shown below,
Hydrogen atom has to be added to the amine part and
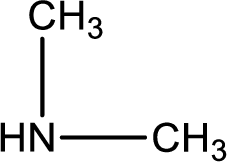
Structure of nitrogen-containing compound that is required to produce the given compound is drawn.
(d)
Interpretation:
Structure of nitrogen-containing compound that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(d)
Answer to Problem 17.136EP
Nitrogen-containing compound that is required was,
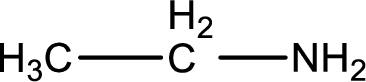
Explanation of Solution
Given structure of compound is,
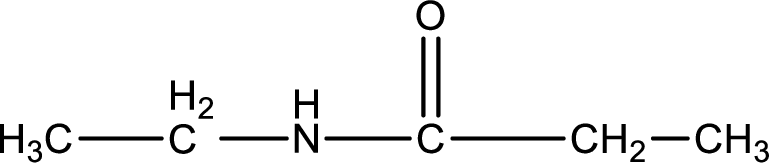
As the nitrogen atom present in the above amide has two hydrogen atoms bonded to it, the amide is a primary amide. Primary amide is produced by the reaction of ammonia with carboxylic acid. The nitrogen-containing compound can be identified as shown below,
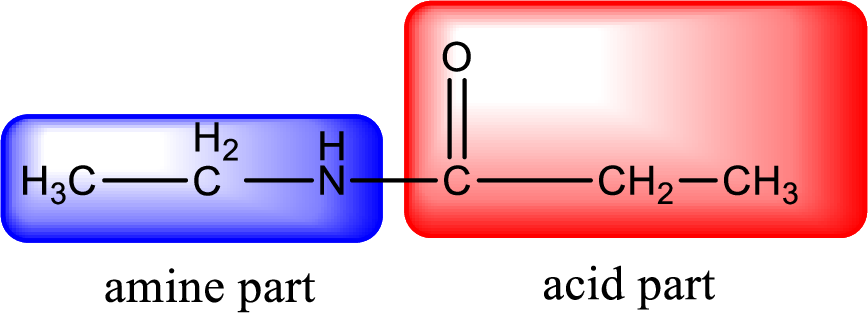
Hydrogen atom has to be added to the amine part and
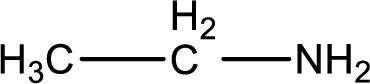
Structure of nitrogen-containing compound that is required to produce the given compound is drawn.
Want to see more full solutions like this?
Chapter 17 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
- (12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- Don't used Ai solutionarrow_forwardIndicate the correct option.a) Graphite conducts electricity, being an isotropic materialb) Graphite is not a conductor of electricityc) Both are falsearrow_forward(f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward
- 1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


