
Interpretation:
The spontaneity of a process depends on several factors; one of the several factors is entropy. When the change in entropy of a process is negative, the process is said to be spontaneous.
Concept introduction:
Entropy is referred to as the 'state of disorder' of a system. Also, entropy is a measure of the energy of atoms and molecules in a process and can be defined in terms of statistical probabilities of a system or in terms of the other
When the change in entropy of a process is negative, the process is said to be spontaneous due to increase randomness of the system. When the change in entropy of a process is positive, the process is said to be non-spontaneous due to decrease in randomness of the system. The change in entropy occurs during phase transitions, precipitation reactions etc.
The change in entropy is defined as the difference between the entropies of the final and initial states:
Given:
Endothermic decomposition of
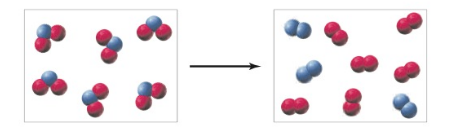
To determine:
To determine the sign (+,-, or 0) of
- for the reaction.
- To determine with explanation if the reaction is more likely to be spontaneous at high temperature or at low temperatures.
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Chemistry (7th Edition)
- Draw the enolate anion and the ester that would be needed to make this product through a Claisen condensation reaction. олarrow_forward1. (10 points). Draw a stepwise detailed mechanism for the following reaction showing all the substitution and the elimination products formed. Label the type of the mechanism (SN1, SN2, E1 or E2). Show stereochemistry when applicable. Br CH3CH2OH H₂O 2. (10 points) 0 Br CH3O Na DMSO Draw the detailed mechanism showing ALL possible products formed including stereochemistry and Label mechanism as SN1, SN2, E1 or E2. Show stereochemistry when applicable.arrow_forwardWhich solution below is considered to have basic character? pH = 2 pOH = 4 pOH = 13 more than one answers is correct. pOH = 5 None of the answers.arrow_forward
- The following titration acid/base curve (pH vs mL of titrant) belongs to which of the following acid-base pairs? 1. strong acid-weak base 2. strong acid-strong base 3. weak acid-strong base 4. weak acid-weak base 5. Can't tell. We need more info.arrow_forwardShow work. Don't give Ai generated solutionarrow_forwardUsing data from Part A, add heat as a reactant or product for each reaction below. heat + NH4NO3 (s) -> NH4+ (aq) + NO3¹- (aq) CaCl2 (s) ㄱ -> Ca2+ (aq) + 2C1¹ (aq) +heat Based on your completed reactions above, draw a reaction coordinate (energy) diagram for both processes. Clear label the reactants and products for each process in the diagram.arrow_forward
- What is the mass percent of a solution containing 27.5 g of table salt and 175 g of water?arrow_forwardIf you have a solution with hydroxide ion ( OH- ) concentration of 0.5350 M, then what would be the pH?arrow_forward0.450 moles of NaCl are dissolved in enough water and make 95.7 ml of solution. Calculate the molarity of the NaCl solution.arrow_forward
- DEC 4 OW Help DH © Macmillan Learning Consider the fatty acid. achieve.macmillanlearning.com 3 ( X Chapter 12 HW - General, Organic, and Biological Chemistry for He H3C-CH2-CH2-CH2-CH2-C-SCOA Resources Hint Draw the carbon-containing products of the fatty acid after one repetition of the ẞ-oxidation pathway. Include the hydrogen atoms in your structures. Select c Draw C 0 H Templates Groups More Erase Q2Q M S A@ Σarrow_forwardOW Help CH © Macmillan Learning achieve.macmillanlearning.com X Chapter 12 HW - General, Organic, and Biological Chemistry for Health: Pancreatic lipases hydrolyze triacylglycerols (triglycerides) that have been emulsified by bile. DEC 4 Resources Hint Modify the triacylglycerol to show the products of its hydrolysis by pancreatic lipase and two water molecules. Select Draw // C о H о Templates 0 in MACBOOK PRO More ال Erase Q2Q A 00 Σarrow_forwardPlease correct answer and don't use hand ratingarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





