
(a)
Interpretation:
The product formed when the given compound gets hydrolyzed by aqueous acid should be determined.
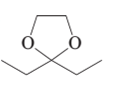
Concept Introduction:
Hemi-acetal or acetal is not very stable. It returns to

(b)
Interpretation:
The product formed when the given compound gets hydrolyzed by aqueous acid should be determined.
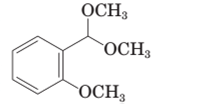
Concept Introduction:
Hemi-acetal or acetal is not very stable. It returns to aldehyde / ketone when a hemi acetal or acetal undergoes hydrolysis in acid medium.

(c)
Interpretation:
The product formed when the given compound gets hydrolyzed by aqueous acid should be determined.
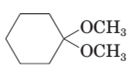
Concept Introduction:
Hemi-acetal or acetal is not very stable. It returns to aldehyde / ketone when a hemi acetal or acetal undergoes hydrolysis in acid medium.

(d)
Interpretation:
The product formed when the given compound gets hydrolyzed by aqueous acid should be determined.
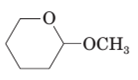
Concept Introduction:
Hemi-acetal or acetal is not very stable. It returns to aldehyde / ketone when a hemi acetal or acetal undergoes hydrolysis in acid medium.

Trending nowThis is a popular solution!

Chapter 16 Solutions
Introduction To General, Organic, And Biochemistry
- 17-47 What is the characteristic structural feature of a hemiacetal? Of an acetal?arrow_forward17-70 What simple chemical test could you use to distinguish between the members of each pair of com pounds? Tell what you would do, what you would expect to observe, and how you would interpret your experimental observation. (a) Benzaldehyde and cyclohexanone (b) Acetaldehyde and acetonearrow_forward17-67 Draw structural formulas for these compounds. (a) 1-Chloro-2-propanone (b) 3-Hydroxybutanal (c) 4-Hydroxy-4-methyl-2-pentanone (d) 3-Methyl-3-phenylbutanal (e) 1,3-Cyclohexanedione (f) 5-Hydroxyhexanalarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,


