
Concept explainers
Write the structure of the principal organic product formed in the reaction of
Sulfuric acid (catalytic amount), heat at
°C
Sulfuric acid (catalytic amount), heat at
°C
Dimethyl sulfoxide (DMSO), oxalyl chloride
Pyridinium chlorochromate (PCC) in dichloromethane
Potassium dichromate
Acetic acid 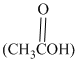 in the presence of dissolved hydrogen chloride
in the presence of dissolved hydrogen chloride
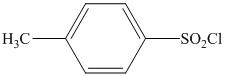 in the presence of pyridine
in the presence of pyridine
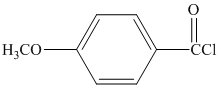 in the presence of pyridine
in the presence of pyridine
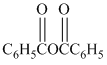 in the presence of pyridine
in the presence of pyridine
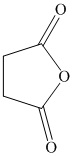 in presence of pyridine
in presence of pyridine
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Organic Chemistry - Standalone book
- In an advanced synthetic chemistry experiment, a researcher prepares a compound, ZY-7, by reacting a ketone (C5H10O) with hydroxylamine (NH2OH), followed by heating in the presence of an acid catalyst. The resulting compound, ZY-7, is then treated with a solution of sodium nitrite (NaNO2) and hydrochloric acid (HCl) at low temperature. Identify the class of compound that ZY-7 most likely belongs to after this series of reactions." A) Amide B) Oxime C) Nitro compound D) Diazonium salt E) Ester Don't use chatgpt please provide valuable answerarrow_forwardIsoamyl acetate is the common name of the substance most responsible for the characteristic odor of bananas. Write a structural formula for isoamyl acetate, given the information that it is an ester in which the carbonyl group bears a methyl substituent and there is a 3-methylbutyl group attached to one of the oxygens.arrow_forwardWrite the structure of the following compounds 5-methyloctane-2,6-diol 2-methylpropane-1,2,3-triol 4-methylhexa-1-en-2,5-diol (2-methyl)hexyl phenyl ether decane-2,4,6,8-tetraol butylpropyl sulfide Dicyclopentyl ether Cyclobutylphenyl ether 2-chlorophenol Cyclopentyl epoxidearrow_forward
- Alkenes can be converted to alcohols by reaction with mercuric acetate to form a β-hydroxyalkylmercury(II) acetate compound, a reaction called oxymercuration. Subsequent reduction with NaBH4 reduces the C–Hg bond to a C–H bond, forming the alkyl alcohol, a reaction called demercuration. Draw the structures of the Hg-containing compound(s) and the final alcohol product(s) formed in the following reaction sequence, omitting byproducts. If applicable, draw hydrogen at a chirality center and indicate stereochemistry via wedge-and-dash bonds.arrow_forwardWrite structural formulas for all ketones with the molecular formula C6H12O and give each its IUPAC name. Which of these ketones are chiral?arrow_forwardArrange these compounds in order of increasing acidity: 2,4-dichlorophenol, phenol, cyclohexanol.arrow_forward
- When bromine is added to two beakers, one containing phenyl isopropyl ether and the other containing cyclohexene, the bromine color in both beakers disappears. What observation could you make while performing this test that would allow you to distinguish the alkene from the aryl ether?arrow_forwardThree products with the molecular formula C6 H4BrCl form when bromobenzene is treated with chlorine, Cl2, in the presence of FeCl3 as a catalyst. Name and draw a structural formula for each product.arrow_forwardCompounds X and Y have the formula C6H12- Both X and Y react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methylpentane. The heat of hydrogenation of X is less than that of Y. X and Y react with HBr to form a mixture of the same bromoalkanes, and they both undergo hydroboration/oxidation to give a mixture of the same alcohols. What is the structure of Y? In cases where there is more than one answer, just draw one. n. n [ ]# ChemDoodleⓇ zaarrow_forward
- Compound A and compound B are different alcohols with the same molecular formula of C4H10O. When reacted with chromic acid (H2CrO4), compound A and compound B produce compound C and compound D, respectively. Compound C has a molecular formula of C4H8O2. Compound E is obtained when compound A reacts with pyridinium chlorochromate (PCC) in a solvent such as dichloromethane (CH2Cl2). Draw the structure of compound A, B, C, D and E.arrow_forwardDraw a structural formula for the major organic product of the following reaction: CH2Cl2, + Br2arrow_forwardEsterication is the reaction of a carboxylic acid (RCOOH) with an alcohol (R'OH) to form an ester (RCOOR') with loss of water. Equation [1] is an example of an intermolecular esterication reaction. Equation [2] is an example of an intramolecular esterication reaction; that is, the carboxylic acid and alcohol are contained in the same starting material, forming a cyclic ester as product. The equilibrium constants for both reactions are given. Explain why Keq is different for these two apparently similar reactions.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
