
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 16.13P
16-13 Classify each amino group as primary, secondary, or tertiary and as aliphatic or
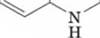
Serotonin
(a neurotransmitter)
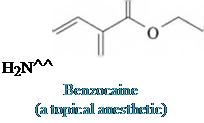
Diphenhydramine
(the hydrochloride salt is
the antihistamine Benadryl)
Lysine
(an amino acid)
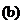
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Complete this table for different amine compounds.
Chemical
propylamine
quaternary
ammonium ion
methylphenylamine
Molecular
formula
C3H9N
C₂H7N
C5H13N
C4H10N
Structural formula
(CH3)3N
(CH3)2NH
CH 3
I
(CH₂) 11
|
H3C(CH2) 11 -N-(CH₂)11CH3
NHCH3
(CH₂)11
CH3
CH3CH2CH2-NH-CH3
+
Classification
Tertiary
Quaternary
Tertiary
Secondary
True or false
The hydroxyl group found in an organic compound is responsible for its basicity.
Aromatic hydrocarbons are considered polar and has high affinity to water.
Acidic drugs are a class of chemical compounds that normally have high hydrophilicity and negative charges.
Amino groups attached to hydrocarbons are considered polar which may be responsible for its solubility.
Lipophilic drugs have a faster rate of absorption than hydrophilic drugs.
Chapter 16 Solutions
Introduction to General, Organic and Biochemistry
Ch. 16.1 - Problem 16-1 How many hydrogen atoms does...Ch. 16.2 - Problem 16-2 Write a structural formula for each...Ch. 16.2 - Prob. 16.3PCh. 16.4 - Problem 16-4 Select the stronger base from each...Ch. 16.5 - Prob. 16.5PCh. 16 - 16-6 Answer true or false. te/7-Butylamine is a 3°...Ch. 16 - Prob. 16.7PCh. 16 - Prob. 16.8PCh. 16 - 16-9 In what way are pyridine and pyrimidine...Ch. 16 - Prob. 16.10P
Ch. 16 - Prob. 16.11PCh. 16 - Prob. 16.12PCh. 16 - 16-13 Classify each amino group as primary,...Ch. 16 - Prob. 16.14PCh. 16 - 16-15 There are eight primary amines with the...Ch. 16 - Prob. 16.16PCh. 16 - 16-17 Propylamine (bp 48°C), ethylmethylamine (bp...Ch. 16 - 16-18 Account for the fact that 1-butanamine (bp...Ch. 16 - 16-19 2-Me thy 1 propane (bp -12°C), 2-propanol...Ch. 16 - Prob. 16.20PCh. 16 - Prob. 16.21PCh. 16 - Prob. 16.22PCh. 16 - Prob. 16.23PCh. 16 - Prob. 16.24PCh. 16 - Prob. 16.25PCh. 16 - 16-26 The p/fb of amphetamine is approximately 3.2...Ch. 16 - 16-27 Guanidine, p/Ca 13.6, is a very strong base,...Ch. 16 - 16-28 Following is the structural formula of...Ch. 16 - Prob. 16.29PCh. 16 - Prob. 16.30PCh. 16 - Prob. 16.31PCh. 16 - 16*32 Many tumors of the breast are correlated...Ch. 16 - Prob. 16.33PCh. 16 - Prob. 16.34PCh. 16 - 16-35 (Chemical Connections 16B ) What is an...Ch. 16 - Prob. 16.36PCh. 16 - Prob. 16.37PCh. 16 - Prob. 16.38PCh. 16 - Prob. 16.39PCh. 16 - Prob. 16.40PCh. 16 - Prob. 16.41PCh. 16 - Prob. 16.42PCh. 16 - Prob. 16.43PCh. 16 - Prob. 16.44PCh. 16 - Prob. 16.45PCh. 16 - 16-46 Arrange these three compounds in order of...Ch. 16 - Prob. 16.47PCh. 16 - Prob. 16.48PCh. 16 - Prob. 16.49PCh. 16 - Prob. 16.50PCh. 16 - Prob. 16.51PCh. 16 - Prob. 16.52PCh. 16 - Prob. 16.53PCh. 16 - 16-54 Several poisonous plants, including Atropa...Ch. 16 - Prob. 16.55PCh. 16 - Prob. 16.56PCh. 16 - Prob. 16.57PCh. 16 - 16-58 Following is a structural formula of...Ch. 16 - Prob. 16.59P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 16-54 Several poisonous plants, including Atropa belladonna, contain the alkaloid atropine. The name “belladonna” (which means “beautiful lady”) probably comes from the fact that Roman women used extracts from this plant to make themselves more attractive. Atropine is widely used by ophthal mologists and optometrists to dilate the pupils for eye examination. Classify the amino group in atropine as primary, secondary, or tertiary. Locate all stereocenters in atropine. Account for the fact that atropine is almost insoluble in water (1 g in 455 mL of cold water) but atropine hydrogen sulfate is very soluble (1 g in 5 mL of cold water). Account for the fact that a dilute aqueous solution of atropine is basic (pH approximately 10.0).arrow_forward16-28 Following is the structural formula of metformin, the hydrochloride salt of which is marketed as the antidiabetic medication Glucophage. Metformin was introduced into clinical practice in the United States in 1995 for the treatment of type 2 diabetes. More than 25 million prescriptions for this drug were written in 2000, making it the most commonly prescribed brand-name diabetes medication in the nation. NH NH H3(\ 3 N N Nh2ch3 h Metformin Complete the Lewis structure for metformin, showing all valence electrons. Which nitrogen is the most likely site of protonation? Draw the structural formula of Glucophage.arrow_forward17-13 Which compounds contain carbonyl groups?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY