
Concept explainers
(a)
Interpretation:
Concept Introduction:
The formula to calculate molar mass from number of moles is given as follows:
The expression to evaluate
(a)
Explanation of Solution
Since in
The formula to evaluate molarity is given as follows:
Substitute
Thus concentration of
The expression to evaluate
Substitute
Hence,
(b)
Interpretation:
Whether solution formed
Concept Introduction:
Refer to part (a).
(b)
Explanation of Solution
Since in
Since in
The balanced equation is given as follows:
As per balanced equation (3) since
Since moles of
Total volume is calculated as follows:
Substitute
Thus concentration of
Substitute
Hence,
(c)
Interpretation:
Whether solution formed
Concept Introduction:
Refer to part (a).
(c)
Explanation of Solution
Since in
Since in
As per balanced equation (3) since
Since moles of
Total volume is calculated as follows:
Substitute
Substitute
Hence,
(d)
Interpretation:
Whether solution formed
Concept Introduction:
Refer to part (a).
(d)
Explanation of Solution
Since in
Since in
As per balanced equation (3) since
Since moles of
Total volume is calculated as follows:
Substitute
Thus concentration of
Substitute
Hence,
(e)
Interpretation:
Whether solution formed
Concept Introduction:
Refer to part (a).
(e)
Explanation of Solution
Since in
Since in
As per balanced equation (3) since
Since moles of
Total volume is calculated as follows:
Substitute
Substitute
Hence,
(f)
Interpretation:
Whether solution formed
Concept Introduction:
Refer to part (a).
(f)
Explanation of Solution
Since in
Since in
As per balanced equation (3) since
Since moles of
Total volume is calculated as follows:
Substitute
Substitute
Hence,
(g)
Interpretation:
Whether solution formed
Concept Introduction:
Refer to part (a).
(g)
Explanation of Solution
Since in
Since in
As per balanced equation (3) since
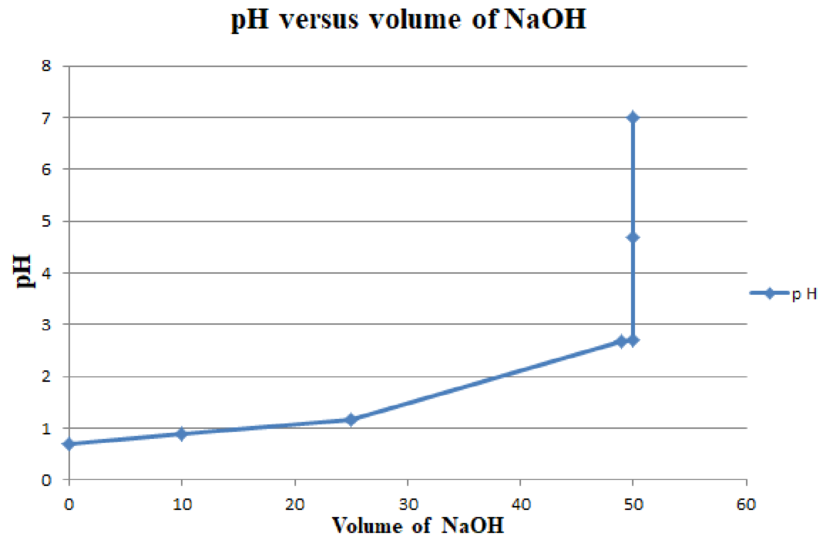
The table for plot of
Plot of
Want to see more full solutions like this?
Chapter 15 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
- Vitamin C is ascorbic acid (HC6H7O6), for which Ka is 8.0105. Calculate the pH of a solution made by dissolvinga 500-mg tablet of pure vitamin C in water anddiluting to 100 mL.arrow_forwardA 10.0-mL solution of 0.780 M NH, is titrated with a 0.260 M HCl solution. Calculate the pH after the following additions of the HCI solution: (а) 0.00 mL (b) 10.0 mL (с) 30.0 mL (d) 40.0 mLarrow_forwardCalculate the pH during the titration of 40.00 mL of 0.1000 M HCl with 0.1000 M NaOH solution after the following additions of base: (a) 28.00 mL (b) 39.50 mL (c) 52.00 mLarrow_forward
- 13. A 60.00 mL sample of 0.075 M sodium benzoate (NaC7H5O2) was titrated with 0.050 M HCl. What is the pH of the solutionafter 10.00 ml of HCl is added?(a) 4.19(b) 5.09(c) 5.74(d) 6.2414. What is the ratio of moles of benzoate (C7H5O2‒) to benzoic acid (HC7H5O2) in the solution that results from thecombination of the NaC7H5O2 and HCl in the problem above?(a) 8(b) 0.125(c) 0.0040(d) 0.00050arrow_forwardWithout detailed calculation and RICE table, determine whether the following solutions are acidic or basic, buffer or not buffer. Explain your answers by identifying the major species and the dominant chemical reaction. (a) 1M NaCl + 1M HCl in equal amounts (b) 1M NH4Cl + 1M NH3 in equal amounts (c) 1M Na2C2O4+ 1M HCl in equal amounts (d) 2M H3PO4+ 3M NaOH in equal amountsarrow_forwardSuppose 1.00 mol of C,H,COOH and 0.500 mol C,H,COONA are added to water and diluted to 1.00 L. The K, of C,H,COOH is 6.3x10-5, a) Calculate the pH of the solution. b) Is the solution in part (a) better at buffering strong acid or strong base? c) Calculate the pH of the resulting solution if 0.10 mol of HCl is added to the solution in part (a).arrow_forward
- 2. Which of the following could be added to 50.0 mL of 0.10 mol L nitric acid solution to change its pH to 3? (A) 450.0 mL of distilled water (B) 450.0 mL of 0.10 mol L-1 nitric acid (C) 4950.0 mL of distilled water (D) 4950.0 mL of 0.10 mol L'nitric acidarrow_forwardThe titration of 50.00 mL of 0.150-M HCl with 0.150-M NaOH (the titrant) is carried out in a chemistry labora- tory. Calculate the pH of the solution after these volumes of the titrant have been added: (a) 0.00 mL (d) 50.00 mL Use the results of your calculations to plot a titration curve for this titration. On the curve indicate the position of the equivalence point. (b) 25.00 mL (e) 50.1 mL (c) 49.9 mL (f) 75.00 mLarrow_forward- What is the pH of each of the following solutions? (a) 0.35 M hydrochloric acid (b) 0.35 M acetic acid (c) 0.035 M acetic acid [Note that the approximate method used in Equation 2.15 will not give a good answer at low Ag.]arrow_forward
- Without detailed calculation and RICE table, determine whether the following solutions are acidic or basic, buffer or not buffer.Explain your answers by identifying the major speciesand the dominate chemical reaction. (a)1M NaCl + 1M HCl in equal amounts (b)1M NH4Cl + 1M NH3in equal amounts (c)1M Na2C2O4+ 1M HCl in equal amounts (d)2M H3PO4+ 3M NaOH in equal amountsarrow_forwardA 25.0 mL solution of 0.100 M CH3CHOOH is titrated with a 0.200 M KOH solution. Calculate the pH after the following additions of the KOH solutions: (a) 0.0 mL (b) 5.0 mL (c) 10.0 mL (d) 12.5 mL (e) 15.0 mLarrow_forwardYou have 75.00 mL of a 0.25 M NaOH solution that you are going to titrate with 0.5M Hal. What is the pH of the resulting solutions after the following additions ? (A) initial pH (B) after 10.50 mL of HCl have been added (C) at the equivalence point (D) after 40.00 mL of HCl have been added (E) what indicator would you choose to signal the endpoint, and WHY?arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning


