
(a)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with
Concept introduction:
Esters can be prepared from the reaction of an alcohol with
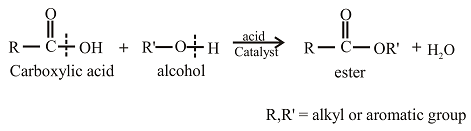
The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
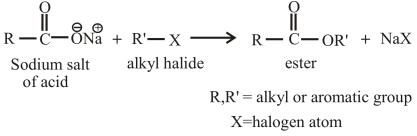
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,
(b)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with alkyl halide for each of the given ester formations.
Concept introduction:
Esters can be prepared from the reaction of an alcohol with carboxylic acid. The general reaction between acid and alcohol can be given as,

The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,

(c)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with alkyl halide for each of the given ester formations.
Concept introduction:
Esters can be prepared from the reaction of an alcohol with carboxylic acid. The general reaction between acid and alcohol can be given as,

The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,

(d)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with alkyl halide for each of the given ester formations.
Concept introduction:
Esters can be prepared from the reaction of an alcohol with carboxylic acid. The general reaction between acid and alcohol can be given as,

The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,

Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
- What will be the final product when 1-butanol is oxidized with Cro, in an acidic medium? Select one: A. butanoic acid B. butanal C. butyl chromate D. butyl butanoatearrow_forwardExplain why acetyl chloride reacts faster with water than acetic anhydride does?arrow_forwardPhosgene (COCl2) was used as a poison gas in World War I. What product would be formed from the reaction of phosgene with each of the following reagents? a. one equivalent of methanol b. excess methanol c. excess propylamine d. excess waterarrow_forward
- 4-Hydroxy- and 5-hydroxyaldehydes exist primarily as cyclic hemiacetals. Draw the structure of the cyclic hemiacetal formed by each of the following: a. 4-hydroxybutanal b. 4-hydroxypentanal c. 5-hydroxypentanal d. 4-hydroxyheptanalarrow_forwardWhen trichloroacetaldehyde is dissolved in water, almost all of it is converted to the hydrate. Chloral hydrate, the product of the reaction, is a sedative that can be lethal. A cocktail laced with it is known—in detective novels,at least—as a “Mickey Finn.” Explain why an aqueous solution of trichloroacetaldehyde is almost all hydrate.arrow_forwardWhen trichloroacetaldehyde is dissolved in water, almost all of it is converted to the hydrate. Chloral hydrate, the product of the reaction, is a sedative that can be lethal. A cocktail laced with it is known—in detective novels, at least—as a “Mickey Finn.” Explain why an aqueous solution of trichloroacetaldehyde is almost all hydrate.arrow_forward
- When phenylbenzoate is heated with aqueous acid solution, the products formed are: A. 2 moles of benzylalcohol B. 1 mole benzoic acid + 1 mole benzylalcohol C. 1 mole benzoic acid + 1 mole phenol D. 2 moles of benzoic acidarrow_forwardHow many products are obtained when the phospholipid below is saponified? a. 4 b. 6 c. 2 d. 3 e. 5arrow_forwardplease explain why phenol is not easily attacked by a positive reagent such as aniline?arrow_forward
- In which of the following solvents will naphthalene dissolve best? A.) A B.) B C.) C D.) Darrow_forwardWhat test will allow you to distinguish between: a. benzyl alcohol and cyclohexanolb. benzyl alcohol and phenol c. cyclohexanol and 1-methylcyclohexanol d. o-cresol and anisolee. benzyl alcohol and anisolearrow_forwardHow many products are obtained when the phospholipid below is saponified? a. 2 b. 6 c. 5 d. 3arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


