
(a)
Interpretation:
The intermolecular interactions that operate in
Concept introduction:
There are three types of interactions through which the molecules are stabilized. They are hydrogen-bonding interactions, the dipole-dipole interactions and the induced dipole interactions. These interactions influence the properties of the compounds like boiling point, melting point and so on.
Answer to Problem 39E
The intermolecular interactions that operate in
Explanation of Solution
In all molecular substances, the intermolecular interactions exist. These interactions are induced dipole interactions, dipole-dipole interactions and hydrogen bonding.
The structure of
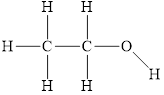
Figure 1
The molecule has electronegative atom
The intermolecular interactions that operate in
(b)
Interpretation:
The intermolecular interactions that operate in
Concept introduction:
There are three types of interactions through which the molecules are stabilized. They are hydrogen-bonding interactions, the dipole-dipole interactions and the induced dipole interactions. These interactions influence the properties of the compounds like boiling point, melting point and so on.
Answer to Problem 39E
The intermolecular interactions that operate in
Explanation of Solution
In all molecular substances, the intermolecular interactions exist. These interactions are induced dipole interactions, dipole-dipole interactions and hydrogen bonding.
The structure of
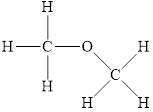
Figure 2
The molecule has electronegative atom
The intermolecular interactions that operate in
(c)
Interpretation:
The intermolecular interactions that operate in
Concept introduction:
There are three types of interactions through which the molecules are stabilized. They are hydrogen-bonding interactions, the dipole-dipole interactions and the induced dipole interactions. These interactions influence the properties of the compounds like boiling point, melting point and so on.
Answer to Problem 39E
The intermolecular interactions that operate in
Explanation of Solution
In all molecular substances, the intermolecular interactions exist. These interactions are induced dipole interactions, dipole-dipole interactions and hydrogen bonding.
The structure of
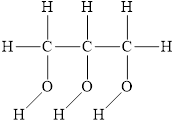
Figure 3
The molecule has electronegative atom
The intermolecular interactions that operate in
(d)
Interpretation:
The intermolecular interactions that operate in
Concept introduction:
There are three types of interactions through which the molecules are stabilized. They are hydrogen-bonding interactions, the dipole-dipole interactions and the induced dipole interactions. These interactions influence the properties of the compounds like boiling point, melting point and so on.
Answer to Problem 39E
The intermolecular interactions that operate in
Explanation of Solution
In all molecular substances, the intermolecular interactions exist. These interactions are induced dipole interactions, dipole-dipole interactions and hydrogen bonding.
The structure of
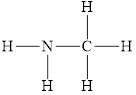
Figure 4
The molecule has electronegative atom
The intermolecular interactions that operate in
(e)
Interpretation:
The intermolecular interactions that operate in
Concept introduction:
There are three types of interactions through which the molecules are stabilized. They are hydrogen-bonding interactions, the dipole-dipole interactions and the induced dipole interactions. These interactions influence the properties of the compounds like boiling point, melting point and so on.
Answer to Problem 39E
The intermolecular interactions that operate in
Explanation of Solution
In all molecular substances, the intermolecular interactions exist. These interactions are induced dipole interactions, dipole-dipole interactions and hydrogen bonding.
The structure of
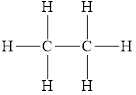
Figure 5
The molecule does not have any has electronegative atom. This means the polarity is not present in the molecule. This molecule can form bond with other molecule through the induced dipole interactions. Therefore, the dominant intermolecular interaction in this molecule is induced dipole interaction.
The intermolecular interactions that operate in
Want to see more full solutions like this?
Chapter 15 Solutions
Introductory Chemistry: An Active Learning Approach
- Hi!! Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year organic chemistry course. ****the solution must include all steps, mechanisms, and intermediate structures as required. Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!! I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!arrow_forwardIn three dimensions, explain the concept of the velocity distribution function of particles within the kinetic theory of gases.arrow_forwardIn the kinetic theory of gases, explain the concept of the velocity distribution function of particles in space.arrow_forward
- In the kinetic theory of gases, explain the concept of the velocity distribution function of particles.arrow_forwardHi!! Please provide a solution that is handwritten. this is an inorganic chemistry question please answer accordindly!! its just one question with parts JUST ONE QUESTION with its parts spread out till part (g), please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures, please DRAW DRAW them on a paper and post clearly!! answer the full question with all calculations step by step EACH PART CLEARLY please thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forwardHi!! Please provide a solution that is handwritten. this is an inorganic chemistry question please answer accordindly!! its just one question with parts JUST ONE QUESTION, please answer EACH part PART A AND PART B!!!!! till the end and dont just provide wordy explanations wherever asked for structures, please DRAW DRAW them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forward
- Hi!! Please provide a solution that is handwritten. this is an inorganic chemistry question please answer accordindly!! its just one question with parts JUST ONE QUESTION, please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures, please DRAW DRAW them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forward8b. Explain, using key intermediates, why the above two products are formed instead of the 1,2-and 1,4- products shown in the reaction below. CIarrow_forward(5pts) Provide the complete arrow pushing mechanism for the chemical transformation depicted below Use proper curved arrow notation that explicitly illustrates all bonds being broken, and all bonds formed in the transformation. Also, be sure to include all lone pairs and formal charges on all atoms involved in the flow of electrons. CH3O H I I CH3O-H H I ① Harrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





