
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 15, Problem 15.4QAP
Interpretation Introduction
(a)
Interpretation:
The compound which has greater fluorescence quantum yield from the following should be selected:
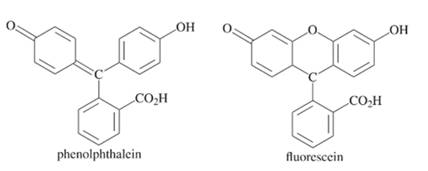
Concept introduction:
There are different cases to check the magnitude of fluorescence quantum yield of the compound. Any compound having these cases will surely have greater fluorescence quantum yield.
Interpretation Introduction
(b)
Interpretation:
The compound which has greater fluorescence quantum yield from the following should be selected:
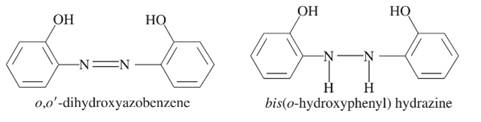
Concept introduction:
There are different scenarios to check whether the magnitude of fluorescence quantum yield of a compound is more or less. Any compound containing these scenarios will surely have more fluorescence quantum yield.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Using the diagram below, draw arrow(s) corresponding to the process of fluorescence. Briefly explain why the emission wavelength via the fluorescence mechanism is longer than the absorbed wavelength.
If the absorbance of 0.482 M copper (II) chloride solution is 0.742 and the path length of light is 1.00 cm, what is the Beer's Law extinction coefficient, epsilon?
A solution of copper(II) sulfate is observed to be blue in color. The maximum absorbance is expected to occur in what wavelength range?
500-560 nm (green)
380-435 nm (violet)
610-750 nm (red)
580-595 nm (yellow)
435-480 nm (blue)
Chapter 15 Solutions
Principles of Instrumental Analysis
Knowledge Booster
Similar questions
- Predict what you expect to observe in the EPRspectrum of a species in which an unpaired electroninteracts with one 14N nucleus (I = 1) and one 1Hnucleus (I = 1/2) if the hyperfine coupling constantsare (a) A(14N) = A(1H)=30 G; (b) A(14N)= 30 G,A(1H)= 10 G.arrow_forwardWhich of the following is true about fluorescence intensity of the molecule? I Fluorescence is observed in planar molecules and its intensity increase with an increase in the number of aromatic rings II When zinc is added to a hydroxyl quinoline solution the fluorescence intensity increased due to the formation of a complex which increases the rigidity of the molecule. III On increasing the intensity of the incident radiation the fluorescence intensity increases IV On increasing the concentration of the fluorigenic molecule, the fluorescence intensity increases. A plot of intensity vs concentration exhibita linear relation indefinitely in all concentration ranges. O a. II,III,IV O b. 1,11,1II,IV O. I,II,III O d. II,I,IV O e. I,II,IVarrow_forwardIn what wavelength range would you expect to find the maximum in the absorption spectrum of the dye “crystal violet”?arrow_forward
- 1. Describe the mechanisms of fluorescence and phosphorescence (include a sketch showing the different transitions). 2. How could you test the proposed mechanisms?arrow_forward2. Draw a diagram of energy levels in the EPR spectrum in each of the following cases and specify the number of transfers. (A) A system with S 5/2 in the absence of zero field splitting (B) A system with S 5/2 with small zero field splitting (C) A system with S 5/2 with large zero field splitting (D) A system with S = 3/2 (for electron spin) in interaction with a nucleus with spin I 3/2arrow_forward2. A combined system is formed from two separate systems of N localized atoms of spin-1/2 each. While the two systems remain separate (a) Find the number of configurations which give a total spin excess S = S, +S, where S, and S, are the excess spins of the separate systems. (b) For what values of S, and S, is this number of configurations maximum?arrow_forward
- When using spin-spin splitting to interpret an NMR spectrum we use the equation 2n + 1 to indicate the number of peaks an NMR absorbance has been split into. What does the “n” stand for?arrow_forwardDetermine the wavelength at which maximum absorption takes place.arrow_forwardDefine fluorescence spectroscopyarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,