
General Chemistry: Atoms First
2nd Edition
ISBN: 9780321809261
Author: John E. McMurry, Robert C. Fay
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 15.37CP
The following pictures represent solutions at various stages in the titration of a weak diprotic acid H2A with aqueous NaOH. (Na+ ions and water molecules have been omitted for clarity.)
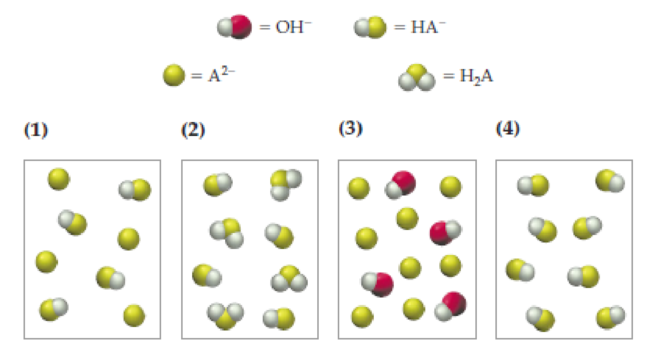
- (a) To which of the following stages do solutions 1–4 correspond?
- (i) Halfway to the first equivalence point
- (ii) At the first equivalence point
- (iii) Halfway between the first and second equivalence points
- (iv) Beyond the second equivalence point
- (b) Which solution has the highest pH? Which has the lowest pH?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Determine the pH during the titration of 59.5 mL of 0.490 M hypochlorous acid (Ką = 3.5×10-8) by 0.490 M NaOH at the following points. (Assume the titration is
done at 25 °C.)
(a) Before the addition of any NaOH
(b) After the addition of 15.0 mL of NaOH
(c) At the half-equivalence point (the titration midpoint)
(d) At the equivalence point
(e) After the addition of 89.3 mL of NaOH
Calculate the pH at the following points in a titration of 40.0 mL of 0.100 M barbituric acid(Ka = 9.8 × 10−5) with 0.100 M KOH.
(a) no KOH added
(b) 20.0 mL of KOH solution added
(c)39.0 mL of KOH solution added
(d) 40.0 mL of KOH solution added
(e) 41.0 mL of KOHSketch an appropriate pH titration curve indicating the buffer region, equivalence point,and excess base region. Why is the pH at the equivalence point not 7.00?
Assume you titrate 20.0 mL of 0.11 M NH3 with 0.10 M HCl.
(a) What is the pH of the NH3 solution before the titration begins?
(b) What is the pH of the equivalence point?
(c) What is the pH at the midpoint of the titration?
(d) Which indicator would you suggest to detect the equivalence point?
(e) Calculate the pH of the solution after adding 5.00, 11.0, 15.0, 20.0,
22.0, and 25.0 mL of the acid. Combine this information with that from
(a) through (c) and plot the titration curve.
Chapter 15 Solutions
General Chemistry: Atoms First
Ch. 15.1 - Write balanced net ionic equations for the...Ch. 15.1 - Write balanced net ionic equations for the...Ch. 15.2 - Calculate the concentrations of all species...Ch. 15.2 - Calculate the pH in a solution prepared by...Ch. 15.2 - Prob. 15.5CPCh. 15.3 - The following pictures represent solutions that...Ch. 15.3 - Calculate the pH of 0.100 L of a buffer solution...Ch. 15.3 - Calculate the change in pH when 0.002 mol of HNO3...Ch. 15.4 - Use the HendersonHasselbalch equation to calculate...Ch. 15.4 - Prob. 15.10P
Ch. 15.4 - Suppose you are performing an experiment that...Ch. 15.4 - Prob. 15.12PCh. 15.6 - A 40.0 mL volume of 0.100 M HCl is titrated with...Ch. 15.6 - A 40.0 mL volume of 0.100 M NaOH is titrated with...Ch. 15.7 - The following pictures represent solutions at...Ch. 15.7 - Consider the titration of 100.0 mL of 0.016 M HOCl...Ch. 15.7 - The following acid-base indicators change color in...Ch. 15.9 - Assume that 40.0 mL of 0.0800 M H2SO3 (Ka1 = 1.5 ...Ch. 15.9 - Assume that 40.0 mL of a 0.0250 M solution of the...Ch. 15.10 - Write the equilibrium-constant expression for Ksp...Ch. 15.11 - A saturated solution of Ca3(PO4)2 has [Ca2+] =...Ch. 15.11 - Prob. 15.22PCh. 15.11 - Which has the greater molar solubility: AgCl with...Ch. 15.11 - Prob. 15.24CPCh. 15.12 - Calculate the molar solubility of MgF2 in 0.10 M...Ch. 15.12 - Which of the following compounds are more soluble...Ch. 15.12 - In an excess of NH3(aq), Cu2+ ion forms a deep...Ch. 15.12 - Silver bromide dissolves in aqueous sodium...Ch. 15.13 - Prob. 15.29PCh. 15.13 - Will a precipitate form on mixing 25 mL of 1.0 ...Ch. 15.14 - Prob. 15.31PCh. 15.15 - Prob. 15.32PCh. 15 - The following pictures represent solutions that...Ch. 15 - The following pictures represent solutions that...Ch. 15 - The strong acid HA is mixed with an equal molar...Ch. 15 - The following pictures represent solutions at...Ch. 15 - The following pictures represent solutions at...Ch. 15 - The following pictures represent solutions at...Ch. 15 - Prob. 15.40CPCh. 15 - Prob. 15.41CPCh. 15 - Prob. 15.42CPCh. 15 - Prob. 15.43CPCh. 15 - Is the pH greater than, equal to, or less than 7...Ch. 15 - Prob. 15.45SPCh. 15 - Which of the following mixtures has the higher pH?...Ch. 15 - Which of the following mixtures has the lower pH?...Ch. 15 - Phenol (C6H5OH, Ka = 1.3 1010) is a weak acid...Ch. 15 - Aniline (C6H5NH2, Kb = 4.3 1010) is a weak base...Ch. 15 - The equilibrium constant Kn for the neutralization...Ch. 15 - The equilibrium constant Kn for the neutralization...Ch. 15 - Prob. 15.52SPCh. 15 - Does the pH increase, decrease, or remain the same...Ch. 15 - Prob. 15.54SPCh. 15 - Calculate the pH of a solution prepared by mixing...Ch. 15 - Prob. 15.56SPCh. 15 - The pH of a solution of NH3 and NH4Br is 8.90....Ch. 15 - Prob. 15.58SPCh. 15 - Prob. 15.59SPCh. 15 - Prob. 15.60SPCh. 15 - Which of the following gives a buffer solution...Ch. 15 - Prob. 15.62SPCh. 15 - Prob. 15.63SPCh. 15 - Calculate the pH of a buffer solution that is 0.20...Ch. 15 - Prob. 15.65SPCh. 15 - Calculate the pH of 0.250 L of a 0.36 M formic...Ch. 15 - Calculate the pH of0.375 L of a 0.18 M acetic...Ch. 15 - Prob. 15.68SPCh. 15 - Use the HendersonHasselbalch equation to calculate...Ch. 15 - Prob. 15.70SPCh. 15 - Give a recipe for preparing a CH3CO2HCH3CO2Na...Ch. 15 - Prob. 15.72SPCh. 15 - Prob. 15.73SPCh. 15 - What is the Ka of the amino acid leucine if it is...Ch. 15 - Prob. 15.75SPCh. 15 - Prob. 15.76SPCh. 15 - Make a rough plot of pH versus milliliters of acid...Ch. 15 - Prob. 15.78SPCh. 15 - Consider the titration of 50.0 mL of 0.116 M NaOH...Ch. 15 - Consider the titration of 40.0 mL of 0.250 M HF...Ch. 15 - A 100.0 mL sample of 0.100 M methylamine (CH3NH2,...Ch. 15 - Prob. 15.82SPCh. 15 - Consider the titration of 25.0 mL of 0.0200 M...Ch. 15 - Prob. 15.84SPCh. 15 - The equivalence point was reached in titrations of...Ch. 15 - Prob. 15.86SPCh. 15 - What is the pH at the equivalence point for the...Ch. 15 - Prob. 15.88SPCh. 15 - Prob. 15.89SPCh. 15 - Prob. 15.90SPCh. 15 - Prob. 15.91SPCh. 15 - Prob. 15.92SPCh. 15 - Prob. 15.93SPCh. 15 - Prob. 15.94SPCh. 15 - Prob. 15.95SPCh. 15 - Prob. 15.96SPCh. 15 - Prob. 15.97SPCh. 15 - Use Le Chteliers principle to explain the...Ch. 15 - Use Le Chteliers principle to predict whether the...Ch. 15 - Calculate the molar solubility of PbCrO4 in:...Ch. 15 - Calculate the molar solubility of SrF2 in:...Ch. 15 - Which of the following compounds are more soluble...Ch. 15 - Which of the following compounds are more soluble...Ch. 15 - Prob. 15.104SPCh. 15 - Is the solubility of Fe(OH)3 increased, decreased,...Ch. 15 - Prob. 15.106SPCh. 15 - Prob. 15.107SPCh. 15 - Prob. 15.108SPCh. 15 - Prob. 15.109SPCh. 15 - Calculate the molar solubility of AgI in: (a)Pure...Ch. 15 - Calculate the molar solubility of Cr(OH)3 in 0.50...Ch. 15 - What compound, if any, will precipitate when 80 mL...Ch. 15 - Prob. 15.113SPCh. 15 - Prob. 15.114SPCh. 15 - In qualitative analysis, Al3+ and Mg2+ are...Ch. 15 - Prob. 15.116SPCh. 15 - Can Co2+ be separated from Zn2+ by bubbling H2S...Ch. 15 - Prob. 15.118SPCh. 15 - Prob. 15.119SPCh. 15 - Prob. 15.120SPCh. 15 - Give a method for separating the following pairs...Ch. 15 - Assume that you have three white solids: NaCl,...Ch. 15 - On the same graph, sketch pH titration curves for...Ch. 15 - Prob. 15.124CHPCh. 15 - Prob. 15.125CHPCh. 15 - A saturated solution of Mg(OH)2 in water has pH =...Ch. 15 - Prob. 15.128CHPCh. 15 - In qualitative analysis, Ag+, Hg22+, and Pb2+ are...Ch. 15 - Calculate the molar solubility of MnS in a 0.30 M...Ch. 15 - Prob. 15.131CHPCh. 15 - Prob. 15.132CHPCh. 15 - Prob. 15.133CHPCh. 15 - Prob. 15.134CHPCh. 15 - Prob. 15.135CHPCh. 15 - A 100.0 mL sample of a solution that is 0.100 M in...Ch. 15 - A 0.0100 mol sample of solid Cd(OH)2 (Ksp = 5.3 ...Ch. 15 - Zinc hydroxide, Zn(OH)2 (Ksp = 4.1 1017), is...Ch. 15 - Prob. 15.139CHPCh. 15 - Prob. 15.140MPCh. 15 - Ethylenediamine (NH2CH2CH2NH2, abbreviated en) is...Ch. 15 - A 40.0 mL sample of a mixture of HCl and H3PO4 was...Ch. 15 - A 1.000 L sample of HCl gas at 25 C and 732.0 mm...Ch. 15 - Prob. 15.144MPCh. 15 - Consider the reaction that occurs on mixing 50.0...Ch. 15 - In qualitative analysis, Ca2+ and Ba2+ are...Ch. 15 - A railroad tank car derails and spills 36 tons of...Ch. 15 - Prob. 15.148MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the pH of a buffer that is 0.150 M in a weak acid and 0.150 M in the acids conjugate base? The acids ionization constant is 6.8 106.arrow_forwardWhich of the acid-base indicators discussed in this chapter would be suitable for the titration of (a) HNO3 with KOH. (b) KOH with acetic acid. (c) HCl with NH3. (d) KOH with HNO2. Explain your answers.arrow_forwardWhat is the pH of the solution obtained by titrating 1.30 g of sodium hydrogen sulfate, NaHSO4, dissolved in 50.0 mL of water with 0.175 M sodium hydroxide until the equivalence point is reached? Assume that any volume change due to adding the sodium hydrogen sulfate or to mixing the solutions is negligible.arrow_forward
- Consider the titration of HF (K a=6.7104) with NaOH. What is the pH when a third of the acid has been neutralized?arrow_forwardWhich of these combinations is the best to buffer the pH at approximately 9? Explain your choice. CH3COOH/NaCH3COO HCl/NaCl NH3/NH4Clarrow_forwardA 25.0-mL sample of hydroxylamine is titrated to the equivalence point with 35.8 mL of 0.150 M HCl. a What was the concentration of the original hydroxylamine solution? b What is the pH at the equivalence point? c Which indicators, bromphenol blue, methyl red, or phenolphthalein, should be used to detect the end point of the titration? Why?arrow_forward
- A solution of weak base is titrated to the equivalence point with a strong acid. Which one of the following statements is most likely to be correct? a The pH of the solution at the equivalence point is 7.0. b The pH of the solution is greater than 13.0. c The pH of the solution is less than 2.0. d The pH of the solution is between 2.0 and 7.0. e The pH of the solution is between 7.0 and 13.0. The reason that best supports my choosing the answer above is a Whenever a solution is titrated with a strong acid, the solution will be very acidic. b Because the solution contains a weak base and the acid (titrant) is used up at the equivalence point, the solution will be basic. c Because the solution contains the conjugate acid of the weak base at the equivalence point, the solution will be acidic.arrow_forwardIdentify the buffer system(s)the conjugate acidbase pair(s)present in a solution that contains equal molar amounts of the following: a. HF, KC2H3O2, NaC2H3O2, and NaF b. HNO3, NaOH, H3PO4, and NaH2PO4arrow_forwardIn each of the following questions, assume that there is no volume change when HCl is added to water in part (a) or the phosphate buffer in part (c). (a) Calculate the pH when 0.091 moles of HCl are added to 1.000 liter of water. (b) What is the difference between the pH of pure water (pH 7.00) and the pH of the solution after HCl was added? (c) Calculate the pH when 0.091 moles of HCl are added to 1.000 liters of a buffer containing 0.352M KH2PO4 and 0.321 M K2HPO4. (d) The pH before the HCl was added is equal to 7.168. What is the difference between the pH before adding the HCl and after adding the HCl?arrow_forward
- Calculate the pH for each of the following cases in the titration of 60.0 mL of 0.250-M hydrobromic acid, HBr(aq), with 0.250 M NaOH: (HBr is a strong acid) (a) before addition of any NaOH, (b) after addition of 30.0 mL of NaOH, (c) after addition of 50.0 mL of NaOH, (d) after addition of 60.0 mL of NaOH, and (e) after addition of 80.0 mL of NaOHarrow_forwardTUTOR Analysis of a Weak Acid-Strong Base Titration Curve Determine the pH during the titration of 63.3 mL of 0.465 M formic acid (K₂ = 1.8×10) by 0.465 M KOH at the following points. (Assume the titration is done at 25 °C.) (a) Before the addition of any KOH (b) After the addition of 16.0 mL of KOH (c) At the half-equivalence point (the titration midpoint) (d) At the equivalence point (e) After the addition of 95.0 mL of KOHarrow_forwardDetermine the pH during the titration of 59.7 mL of 0.392 M hypochlorous acid (K₂ = 3.5×10-8) by 0.392 M KOH at the following points. (Assume the titration is done at 25 °C.) (a) Before the addition of any KOH (b) After the addition of 13.0 mL of KOH (c) At the half-equivalence point (the titration midpoint) (d) At the equivalence point (e) After the addition of 89.6 mL of KOH Submit Show Approach Show Tutor Stepsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY