
Concept explainers
(a)
Interpretation: The class of given cycloocta-1,4-diene as isolated, conjugated, cumulated, or a combination is to be stated.
Concept introduction: Conjugated systems have alternate single and double bonds that results in lower the energy and increase the stability of a molecule. Isolated dienes or polyenes are the compounds that comprise double bonds at the large distances separated by more than one single bond, whereas cumulated dienes or polyenes are the compounds that do not possess any single bond in between the two double bonds.
(a)
Answer to Problem 15.24SP
The given compound is classified as isolated diene.
Explanation of Solution
The structure of given diene is shown in figure 1.
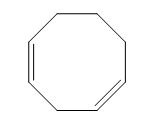
Figure 1
It is conferred from the above structure of
(b)
Interpretation: The class of given cycloocta-1,3-diene as isolated, conjugated, cumulated, or a combination is to be stated.
Concept introduction: Conjugated systems have alternate single and double bonds that results in lower the energy and increase the stability of a molecule. Isolated dienes or polyenes are the compounds that comprise double bonds at the large distances separated by more than one single bond, whereas cumulated dienes or polyenes are the compounds that do not possess any single bond in between the two double bonds.
(b)
Answer to Problem 15.24SP
The given compound is classified as conjugated diene.
Explanation of Solution
The structure of given diene is shown in figure 2.
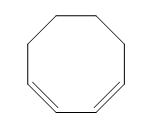
Figure 2
It is conferred from the above structure of
(c)
Interpretation: The class of given cycloocta-1,2-diene as isolated, conjugated, cumulated, or a combination is to be stated.
Concept introduction: Conjugated systems have alternate single and double bonds that results in lower the energy and increase the stability of a molecule. Isolated dienes or polyenes are the compounds that comprise double bonds at the large distances separated by more than one single bond, whereas cumulated dienes or polyenes are the compounds that do not possess any single bond in between the two double bonds.
(c)
Answer to Problem 15.24SP
The given compound is classified as cumulated diene.
Explanation of Solution
The structure of given diene is shown in figure 3.
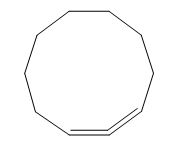
Figure 3
It is conferred from the above structure of
(d)
Interpretation: The class of given cycloocta-1,2,5,7-tetraene as isolated, conjugated, cumulated, or a combination is to be stated.
Concept introduction: Conjugated systems have alternate single and double bonds that results in lower the energy and increase the stability of a molecule. Isolated dienes or polyenes are the compounds that comprise double bonds at the large distances separated by more than one single bond, whereas cumulated dienes or polyenes are the compounds that do not possess any single bond in between the two double bonds.
(d)
Answer to Problem 15.24SP
The given compound is classified as conjugated polyene.
Explanation of Solution
The structure of given polyene is shown in figure 4.
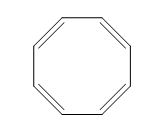
Figure 4
It is conferred from the above structure of
(e)
Interpretation: The class of given cyclohexa-1,3,5-triene as isolated, conjugated, cumulated, or a combination is to be stated.
Concept introduction: Conjugated systems have alternate single and double bonds that results in lower the energy and increase the stability of a molecule. Isolated dienes or polyenes are the compounds that comprise double bonds at the large distances separated by more than one single bond, whereas cumulated dienes or polyenes are the compounds that do not possess any single bond in between the two double bonds.
(e)
Answer to Problem 15.24SP
The given compound is classified as conjugated polyene.
Explanation of Solution
The structure of the given polyene is shown in figure 5.
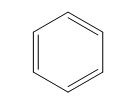
Figure 5
It is conferred from the above structure of
(f)
Interpretation: The class of given penta-1,2-4-triene as isolated, conjugated, cumulated, or a combination is to be stated.
Concept introduction: Conjugated systems have alternate single and double bonds that results in lower the energy and increase the stability of a molecule. Isolated dienes or polyenes are the compounds that comprise double bonds at the large distances separated by more than one single bond, whereas cumulated dienes or polyenes are the compounds that do not possess any single bond in between the two double bonds.
(f)
Answer to Problem 15.24SP
The given compound is classified as cumulated and conjugated polyene.
Explanation of Solution
The structure of given polyene is shown in figure 6.
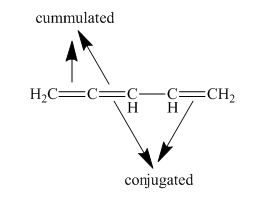
Figure 6
It is conferred from the above structure of
Want to see more full solutions like this?
Chapter 15 Solutions
ORGANIC CHEMISTRY
- Draw structures corresponding to the following IUPAC names: a. 2-methylhexa-1,5-diene b. 3,4-diisopropy;-2,5-dimethyl-3-hexene c. 3-ethyl-2,2-dimethylhept-3-ene d. (4E)-2,4-dimethyl-1,4-hexadiene e. 2,3,3-trimethylocta-1,4,6-triene f. (3E,5Z)-2,6-dimethyl-1,3,5,7- octatetraenearrow_forwardDraw the skeletal structures for the compounds in a. (Z)-1,3,5-tribromo-2-pentene b. (Z)-3-methyl-2-heptenec. (E)-1,2-dibromo-3-isopropyl-2-hexene d. vinyl bromidee. 1,2-dimethylcyclopentene f. diallylaminearrow_forwardDraw the structures for penta-1,4-diene and cyclopenta-1,3-diene.arrow_forward
- 1. Draw structures for a) (2E,4Z) - hepta-2,4-diene b) (S) - 2-bromobutanearrow_forward8. Deformation behavior of a thermoplastic plastic above glass transition temperature a. viscous fluid b. brittle C. leathery and then rubbery d. glassy a. Polymer b. Monomer c. plastic d. synthetic 10. Stages involve in addition polymerization EXCEPT a. Propagation b. Catalyst C. termination d. initiation abioe onarrow_forwardClassify the following dienes and polyenes as isolated, conjugated, cumulated, or some combination of theseclassifications. octa-1,4-dienearrow_forward
- For Nos. 63-70 Choose: A -if the indicated property/behavior in item I is greater than in item II B-if the indicated property/behavior in item I is less than in item II. C- if the indicated property/behavior is equal in item I and item II. D- if the indicated data is insufficient to make a comparison. 63. Reactivity toward Nucleophilic Addition Reaction I. FCH₂CHO II. CH3CHOarrow_forwardNaturally occurring carotene primarily exists in two different forms, called a-carotene and B-carotene. Which has the longer-wavelength UV-vis absorption? Explain. a-Carotene B-Carotenearrow_forward12. Which of the following reactions is classified under addition reactions? A. Bromination of Benzene B. Hydrogenation of 2-butene C. Friedel Crafts Alkylation of Benzene D. Nitration of Toluenearrow_forward
- Which of the statements given below is false? A. It is the reaction reaction between an alkene and a halogen. B. The reaction of an olefin sample with bromine in carbon tetrachloride is an addition reaction. A. Octanol is soluble in ligroin, while it is insoluble in water. D. An olefin sample will decolorize Brominated water, while an alkane sample will cause the yellow orange color to remain. E. By determining the density of hexane and toluene, which are an example of a hydrocarbon, by pictogram, we can obtain information about their physical properties.arrow_forwardDraw the structures of:a. (E)-1-bromo-2-methyl-2-buteneb. (Z)-3-isopropyl-2-heptenearrow_forwardRefer to the equation below to answer questionsWhat is the expected arrangement of the bromine atoms relative to each other among the carbon involvedin pi bonding?A. anti-conformationB. syn-conformationC. trans-configurationD. cis-configurationarrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning



