
The description of the energy changes of the acetic acid from
Explanation of Solution
Introduction:
The acetic acid undergoes either phase change or a rise in temperature due to heating. The latent heat causes the phase change and specific heat causes the rise in temperature.
The aceticacid below the temperature remains in the solid phase. The heating of the acetic acid causes the rise in temperature of the acetic acid. The melting point of the acetic acid is 17 degrees Celsius, so the phase of the acetic acid starts to change from solid to liquid at
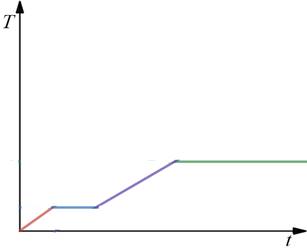
Figure (A)
After the phase change at
Further heating beyond
Conclusion:
Thus, the blue line, the green line shows the phase change and the red line, violet line in the graph shows the rise in temperature of acetic acid due to heating.
Chapter 14 Solutions
Glencoe Physical Science 2012 Student Edition (Glencoe Science) (McGraw-Hill Education)
Additional Science Textbook Solutions
Campbell Biology (11th Edition)
Organic Chemistry (8th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Microbiology: An Introduction
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Chemistry & Chemical Reactivity
- No Chatgpt please will upvote harrow_forwardHelicobacter pylori (H. pylori) is a helically-shaped bacterium that is usually found in the stomach. It burrows through the gastric mucous lining to establish an infection in the stomach's epithelial cells (see photo). Approximately 90% of the people infected with H. pylori will never experience symptoms. Others may develop peptic ulcers and show symptoms of chronic gastritis. The method of motility of H. pylori is a prokaryotic flagellum attached to the back of the bacterium that rigidly rotates like a propeller on a ship. The flagellum is composed of proteins and is approximately 40.0 nm in diameter and can reach rotation speeds as high as 1.50 x 103 rpm. If the speed of the bacterium is 10.0 μm/s, how far has it moved in the time it takes the flagellum to rotate through an angular displacement of 5.00 * 10² rad? Zina Deretsky, National Science Foundation/Flickr H. PYLORI CROSSING MUCUS LAYER OF STOMACH H.pylori Gastric Epithelial mucin cells gel Number i 318 Units um H.pylori…arrow_forwardT1. Calculate what is the received frequency when the car drives away from the radar antenna at a speed v of a) 1 m/s ( = 3.6 km/h), b) 10 m/s ( = 36 km/h), c) 30 m /s ( = 108 km/h) . The radar transmission frequency f is 24.125 GHz = 24.125*10^9 Hz, about 24 GHz. Speed of light 2.998 *10^8 m/s.arrow_forward
- 3. A measurement taken from the UW Jacobson Observatory (Latitude: 47.660503°, Longitude: -122.309424°, Altitude: 220.00 feet) when its local sidereal time is 120.00° makes the following observations of a space object (Based on Curtis Problems 5.12 + 5.13): Azimuth: 225.00° Azimuth rate: 2.0000°/s. Elevation: 75.000° Elevation rate: -0.5000°/s Range: 1500.0 km Range rate: -1.0000 km/s a. What are the r & v vectors (the state vector) in geocentric coordinates? (Answer r = [-2503.47 v = [17.298 4885.2 5.920 5577.6] -2.663]) b. Calculate the orbital elements of the satellite. (For your thoughts: what type of object would this be?) (Partial Answer e = 5.5876, 0=-13.74°) Tip: use Curtis algorithms 5.4 and 4.2.arrow_forwardConsider an isotope with an atomic number of (2(5+4)) and a mass number of (4(5+4)+2). Using the atomic masses given in the attached table, calculate the binding energy per nucleon for this isotope. Give your answer in MeV/nucleon and with 4 significant figures.arrow_forwardA: VR= 2.4 cm (0.1 V/cm) = 0.24 V What do Vector B an C represent and what are their magnitudesarrow_forward
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





