
Chemistry: Atoms First
3rd Edition
ISBN: 9781260083736
Author: Burdge
Publisher: MCGRAW-HILL HIGHER EDUCATION
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1.4, Problem 7PPC
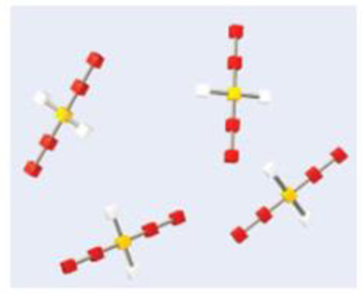
The diagram contains several objects that are constructed using colored blocks and grey connectors. Note that each of the objects is essentially identical, consisting of the same number and arrangement of blocks and connectors. Give the appropriate conversion factor for each of the specified operations.
(a) We know the number of objects and wish to determine the number of red blocks.
(b) We know the number of yellow blocks and wish to determine the number of objects.
(c) We know the number of yellow blocks and wish to determine the number of white blocks.
(d) We know the number of grey connectors and wish to determine the number of yellow blocks.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
11 of 11
I Review | Constants Periodic Table
Suppose that you want to find the equation for a line that passes through the two points
(0,3) and (4, 9). What is the slope of this line?
Express your answer numerically.
> View Available Hint(s)
Πνα ΑΣΦ
?
m =
Submit
rt C Complete previous part(s)
9:41 PM
36%
please help me with this i need answer at the moment, kindly show the full conversion. (1)
Chemistry
(b)
A sampling plan is necessary to ensure that a laboratory sample is truly a
representative of the entire population.
(i)
Describe the goals of the sampling plan.
"It is possible to determine whether the population is homogeneous or
heterogeneous if a single sample is collected and analyzed." Comment
on this statement.
(ii)
Chapter 1 Solutions
Chemistry: Atoms First
Ch. 1.2 - illustrates conversions between these two...Ch. 1.2 - Prob. 1PPACh. 1.2 - According to the website of the National...Ch. 1.2 - If a single degree on the Celsius scale is...Ch. 1.2 - A body temperature above 39C constitutes a high...Ch. 1.2 - The average temperature at the summit of Mt....Ch. 1.2 - Prob. 2PPBCh. 1.2 - If a single degree on the Fahrenheit scale is...Ch. 1.2 - Prob. 1.3WECh. 1.2 - Given that 20.0 mL of mercury has a mass of 272 g....
Ch. 1.2 - Prob. 3PPBCh. 1.2 - Using the picture of the graduated cylinder and...Ch. 1.3 - Determine the number of significant figures in the...Ch. 1.3 - Determine the number of significant figures in the...Ch. 1.3 - Using scientific notation, express the number one...Ch. 1.3 - Perform the following arithmetic operations and...Ch. 1.3 - Perform the following arithmetic operations, and...Ch. 1.3 - Prob. 5PPBCh. 1.3 - Prob. 1.6WECh. 1.3 - Prob. 6PPACh. 1.3 - Prob. 6PPBCh. 1.3 - Several pieces of aluminum metal with a total mass...Ch. 1.4 - The Food and Drug Administration (FDA) recommends...Ch. 1.4 - The American Heart Association recommends that...Ch. 1.4 - A gold nugget has a mass of 0.9347 oz. What is its...Ch. 1.4 - The diagram contains several objects that are...Ch. 1.4 - Prob. 1.8WECh. 1.4 - Prob. 8PPACh. 1.4 - The density of mercury is 13.6 g/cm3. What is its...Ch. 1.4 - Each diagram [(i) or (ii)] shows the objects...Ch. 1 - Prob. 1.1QPCh. 1 - Explain what is meant by the scientific method.Ch. 1 - What is the difference between a hypothesis and a...Ch. 1 - Classily each of the following statements as a...Ch. 1 - Classify each of the following statements as a...Ch. 1 - Name the SI base units that are important in...Ch. 1 - Write the numbers represented by the following...Ch. 1 - What units do chemists normally use for the...Ch. 1 - What is the difference between mass and weight? If...Ch. 1 - Prob. 1.10QPCh. 1 - Prob. 1.11QPCh. 1 - Prob. 1.12QPCh. 1 - Prob. 1.13QPCh. 1 - Prob. 1.14QPCh. 1 - The density of water at 40C is 0.992 g/mL. What is...Ch. 1 - Prob. 1.16QPCh. 1 - Prob. 1.17QPCh. 1 - Prob. 1.18QPCh. 1 - Prob. 1.19QPCh. 1 - Prob. 1.20QPCh. 1 - Indicate which of the following numbers is an...Ch. 1 - Prob. 1.22QPCh. 1 - Distinguish between the terms accuracy and...Ch. 1 - Express the following numbers in scientific...Ch. 1 - Prob. 1.25QPCh. 1 - Prob. 1.26QPCh. 1 - Express the answers to the following calculations...Ch. 1 - Determine the number of significant figures in...Ch. 1 - Prob. 1.29QPCh. 1 - Carry out the following operations as if they were...Ch. 1 - Prob. 1.31QPCh. 1 - Three students (A, B, and C) are asked to...Ch. 1 - Prob. 1.33QPCh. 1 - Prob. 1.34QPCh. 1 - Prob. 1.35QPCh. 1 - The density of the metal bar shown is 8.16 g/cm3....Ch. 1 - The following shows an experiment used to...Ch. 1 - Prob. 1.38QPCh. 1 - Prob. 1.39QPCh. 1 - Prob. 1.40QPCh. 1 - Carry out the following conversions: (a) 1.1 1022...Ch. 1 - The average speed of helium at 25C is 1255 m/s....Ch. 1 - Prob. 1.43QPCh. 1 - Prob. 1.44QPCh. 1 - Prob. 1.45QPCh. 1 - Prob. 1.46QPCh. 1 - Prob. 1.47QPCh. 1 - Prob. 1.48QPCh. 1 - Prob. 1.49QPCh. 1 - Prob. 1.50QPCh. 1 - Prob. 1.51QPCh. 1 - Prob. 1.52QPCh. 1 - The density of ammonia gas under certain...Ch. 1 - Prob. 1.54QPCh. 1 - Prob. 1.55QPCh. 1 - Prob. 1.56QPCh. 1 - Prob. 1.57QPCh. 1 - Classify each of the following as a pure...Ch. 1 - What is the difference between a qualitative...Ch. 1 - Prob. 1.60QPCh. 1 - Prob. 1.61QPCh. 1 - Determine which of the following properties are...Ch. 1 - Prob. 1.63QPCh. 1 - Determine whether the following statements...Ch. 1 - Determine whether each of the following describes...Ch. 1 - Determine whether each of the following describes...Ch. 1 - ADDITIONAL PROBLEMS 1.67 Using the appropriate...Ch. 1 - Prob. 1.68QPCh. 1 - Winch of the following statements describe...Ch. 1 - Prob. 1.70QPCh. 1 - Prob. 1.71QPCh. 1 - In determining the density of a rectangular metal...Ch. 1 - Prob. 1.73QPCh. 1 - Prob. 1.74QPCh. 1 - Prob. 1.75QPCh. 1 - Prob. 1.76QPCh. 1 - A piece of platinum metal weighing 234.0 g is...Ch. 1 - The experiment described in Problem 1.77 is a...Ch. 1 - A copper sphere has a mass of 2.17 103 g. and its...Ch. 1 - Lithium has a very low density (density = 0.53...Ch. 1 - Prob. 1.81QPCh. 1 - Vanillin (used to flavor vanilla ice cream and...Ch. 1 - Prob. 1.83QPCh. 1 - Prob. 1.84QPCh. 1 - Prob. 1.85QPCh. 1 - Prob. 1.86QPCh. 1 - Prob. 1.87QPCh. 1 - Magnesium is used in alloys, in batteries, and in...Ch. 1 - Prob. 1.89QPCh. 1 - The surface area and average depth of the Pacific...Ch. 1 - Calculate the percent error for the following...Ch. 1 - Prob. 1.92QPCh. 1 - Chalcopyrite contains 34.63 percent copper by...Ch. 1 - Prob. 1.94QPCh. 1 - One gallon of gasoline in an automobile's engine...Ch. 1 - Prob. 1.96QPCh. 1 - The worlds total petroleum reserve is estimated at...Ch. 1 - Prob. 1.98QPCh. 1 - Prob. 1.99QPCh. 1 - Chlorine is used to disinfect swimming pools. The...Ch. 1 - Prob. 1.101QPCh. 1 - Prob. 1.102QPCh. 1 - Prob. 1.103QPCh. 1 - Prob. 1.104QPCh. 1 - Prob. 1.105QPCh. 1 - Prob. 1.106QPCh. 1 - Prob. 1.107QPCh. 1 - Prob. 1.108QPCh. 1 - Prob. 1.109QPCh. 1 - Prob. 1.110QPCh. 1 - In January 2009, the National Aeronautics and...Ch. 1 - Prob. 1.112QPCh. 1 - Prob. 1.113QPCh. 1 - Prob. 1.114QPCh. 1 - Prob. 1.115QPCh. 1 - The composition of pennies has changed over the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 42.How many of the following should be included in a good hypothesis? (i) A prediction of the results of the experiment (ii) A step-by-step walkthrough of how to do the experiment with specific references to amounts of reactants, glassware used, and other experimental details (iii) A summation of the data collected after doing the experiment, with comments on accuracy and precision if possible. (iv) A detailed list of chemicals that will be used (v) Waste disposal procedures (vi) A brief scientific explanation of the predicted results. Said another way, the hypothesis should include a scientific explanation of why the predicted results are expected. A. 1 B. 2 C. 3 D. 4 E. 5arrow_forwardTwo students were tasked to weigh 100 mg of sodium chloride (NaCI). Student A pressed the tare button after weighing the weighing paper and obtained the following data. What is the mass (g) of NaCl from Student A? Mass of weighing paper (g) 0.0011 Mass of weighing paper + NaCl (g) 0.1050 Mass of the NaCI (g) Your answer While Student B did not press the tare button after weighing the weighing paper and obtained the following data. What is the mass (g) of NaCl from Student B? * Mass of weighing paper (g) 0.0011 Mass of weighing paper + NaCI (g) 0.1010 Mass of the NaCI (g) Your answerarrow_forward221.2+26.7+402.9arrow_forward
- Dimensional Analysis is a way of doing numerical "book-keeping" when converting quantities or performing calculations. • When converting quantities from one unit to another, conversion factors are used. Solving with Dimensional Analysis and Multiple Units: If I am in Canada where the price of gas is $1.022 USD·L1, how much will it cost me to fill up my gas tank if I travelled 125 km? • Let's also assume that my car gets an average of 30.0 miles/gallon.arrow_forwardA researcher would like to determine whether there is any relationship between students’ grades and where they choose to sit in the classroom. Specifically, the researcher suspects that the better students choose to sit in the front of the room. To test this hypothesis, the researcher asks her colleagues to help identify a sample of n = 100 students who all sit in the front row in at least one class. At the end of the semester, the grades are obtained for these students and the average grade point average is M = 3.25. For the same semester, the average grade point average for the entire college is μ = 2.95 with σ = 1.10. Use a two-tailed test with α = .01 to determine whether students who sit in the front of the classroom have significantly different grade point averages than other students.NOTICE that you are asked to use α = .01! A) sig., p<.01 B) N.S. ("not significant"), p>.01 C) sig., p>.01 D) N.S., p<.01arrow_forward1) State your interpretation of the meaning of the slope for the first graph you prepared of Volume of Steel Nuts (mL) versus Number of Steel Nuts. Hint: Understanding the meaning of a slope involves several factors: the units which are a ratio of the y axis units over the x axis units, the interpretation of the slope as “number of the y axis units for every one of the x axis units” and the concept that constructing the best line or curve that follows the trend in the data is a sort of averaging of the random error throughout the data.arrow_forward
- While creating a piece of art work, a student becomes curious about the ink he is using to create his art. He wonders if the in is a pure substance or a mixture. The ink appears to be uniform throughout, but separates when a chromatography experimer is performed on it, leaving multiple marks of different colors along the chromatography paper. Using his observations of the properties of the ink, the student can classify it as a heterogeneous mixture. solution. compound. element.arrow_forwardI am told that when determining the number of significant figures, if the decimal place is absent, then you start counting from the first nonzero number on the right side and continue counting to the left until you reach the last digit. However, if the decimal place is present, start counting the first nonzero number from the left and continue counting to the right until the final digit has been reached. For example, the number 50 only has two significant figures, while the number 50.00 has four significant figures. However, how does the number 50 have two significant figures? If I have to start countingfrom the right because of the absence of a decimal place, and I have to begin with the first non zero number, I would have to start from 5 and there is nowhere to count further to the left. How does the number 50 have two significant figures?arrow_forwardFind numerical value: Report your answer in proper Sig. Figs. i) (5.6 × 104)+(7.89 x 102). Show Your Working Here: 1.00×105 ii) 8.00 ? Show Your Working Here:arrow_forward
- Compute (4.29 x 105) · (1.89×10-4). Express your answer to three digits.arrow_forwardThe mass of an iron nail is measured before and after being placed in a beaker of water for 2 days. It is found that 0.059 g of iron (3) oxide (rust) was produced over the 2-day period. What mass of iron in the nail reacted with the water? Assume the nail is pure ironarrow_forwardAdd mathematical symbols (+, -, *, /, v, !, etc.) to make the following expressions true. For instance, 1 1 1=6 would become valid with these adjustments: (1 +1+ 1)! = 6 2 2 2= 6 555=6 3 33= 6 6 6 6= 6 4 4 4= 6 777=6arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Measurement and Significant Figures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Gn97hpEkTiM;License: Standard YouTube License, CC-BY
Trigonometry: Radians & Degrees (Section 3.2); Author: Math TV with Professor V;https://www.youtube.com/watch?v=U5a9e1J_V1Y;License: Standard YouTube License, CC-BY