
Organic Chemistry (8th Edition)
8th Edition
ISBN: 9780134042282
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
thumb_up100%
Chapter 14, Problem 49P
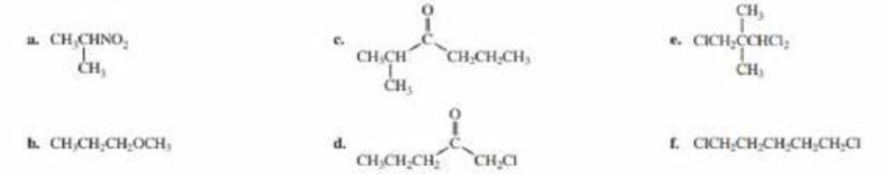
Label each set of chemically equivalent protons, using a for the set that will be at the lowest frequency in the 1H NMR spectrum, b for the next lowest, and so on. Indicate the multiplicity of each signal.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Don't used Ai solution
reaction scheme for C39H4202
Hydrogenation of Alkyne (Alkyne to
Alkene)
show reaction (drawing) please
Give detailed mechanism Solution with explanation needed. Don't give Ai generated solution
Chapter 14 Solutions
Organic Chemistry (8th Edition)
Ch. 14.1 - Prob. 1PCh. 14.1 - Prob. 2PCh. 14.4 - How many signals would you expect to see in the 1H...Ch. 14.4 - How many signals would you expect to see in the 1H...Ch. 14.4 - How could you distinguish the 1H NMR spectra of...Ch. 14.4 - Draw an isomer of dichlorocyclopropane that gives...Ch. 14.5 - Prob. 7PCh. 14.5 - Prob. 8PCh. 14.5 - Prob. 9PCh. 14.5 - Where would you expect to find the 1H NMR signal...
Ch. 14.6 - Prob. 11PCh. 14.7 - Prob. 12PCh. 14.7 - Prob. 13PCh. 14.7 - Without referring to Table 14.1, label the proton...Ch. 14.8 - [18]-Annulene shows two signals in its 1H NMR...Ch. 14.9 - How would integration distinguish the 1H NMR...Ch. 14.9 - Which of the following compounds is responsible...Ch. 14.10 - Prob. 19PCh. 14.10 - Explain how the following compounds, each with the...Ch. 14.10 - The 1H NMR spectra of two carboxylic acids with...Ch. 14.11 - Draw a diagram like the one shown in Figure 14.12...Ch. 14.12 - Indicate the number of signals and the...Ch. 14.12 - Explain the relative chemical shifts of the...Ch. 14.12 - How can their 1H NMR spectra distinguish the...Ch. 14.12 - Identify each compound from its molecular formula...Ch. 14.12 - Predict the splitting patterns for the signals...Ch. 14.12 - Describe the 1H NMR spectrum you would expect for...Ch. 14.12 - Propose structures that are consistent with the...Ch. 14.13 - Prob. 30PCh. 14.13 - Identify the compound with molecular formula...Ch. 14.14 - Prob. 32PCh. 14.15 - a. For the following compounds, which pairs of...Ch. 14.15 - How would the 1H NMR spectra for the four...Ch. 14.17 - Explain why the chemical shift of the OH proton of...Ch. 14.17 - Prob. 38PCh. 14.17 - Prob. 39PCh. 14.17 - Prob. 40PCh. 14.20 - Answer the following questions for each compound:...Ch. 14.20 - Prob. 42PCh. 14.20 - How can 1,2-, 1,3-, and 1,4-dinitrobenzene be...Ch. 14.20 - Identify each compound below from its molecular...Ch. 14.22 - Prob. 45PCh. 14.22 - What does cross peak X in Figure 14.34 tell you?Ch. 14 - Prob. 47PCh. 14 - Draw a spitting diagram for the Hb proton and give...Ch. 14 - Label each set of chemically equivalent protons,...Ch. 14 - Determine the ratios of the chemically...Ch. 14 - How can 1H NMR distinguish between the compounds...Ch. 14 - Prob. 52PCh. 14 - Match each of the 1H NMR spectra with one of the...Ch. 14 - The 1H NMR spectra of three isomers with molecular...Ch. 14 - Prob. 55PCh. 14 - Prob. 56PCh. 14 - Compound A, with molecular formula C4H9Cl, shows...Ch. 14 - Would it be better to use 1H NMR or 13C NMR...Ch. 14 - There are four esters with molecular formula...Ch. 14 - Identify the compound with molecular formula C6H14...Ch. 14 - An alkyl halide reacts with an alkoxide ion to...Ch. 14 - The 1H NMR spectra of three isomers with molecular...Ch. 14 - Identity each of the following compounds from its...Ch. 14 - Identity each of the following compounds from its...Ch. 14 - Prob. 65PCh. 14 - How can the signals in the 6.5 to 8.1 ppm region...Ch. 14 - The 1H NMR spectra of two compounds, each with...Ch. 14 - Draw a splitting diagram for the Hb proton if Jbc...Ch. 14 - Sketch the following spectra that would be...Ch. 14 - How can 1H NMR be used to prove that the addition...Ch. 14 - Identity each of the following compounds from its...Ch. 14 - Dr. N. M. Arr was called in to help analyze the 1H...Ch. 14 - Calculate the amount of energy (in calories)...Ch. 14 - The following 1H NMR spectra are four compounds,...Ch. 14 - When compound A (C5H12O) is treated with HBr, it...Ch. 14 - Identity the compound with molecular formula...Ch. 14 - Identity each of the following compounds from its...Ch. 14 - Prob. 78PCh. 14 - Identify each of the following compounds from its...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY