
Concept explainers
To enhance the effective surface, and hence the chemical reaction rate, catalytic surfaces often take the form of porous solids. One such solid may be visualized as consisting of a large number of cylindrical pores, each of diameter D and length L. 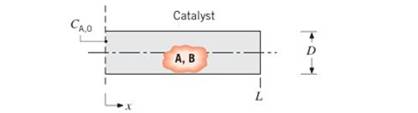
Consider conditions involving a gaseous mixture of A and B for which species A is chemically consumed at the catalytic surface. The reaction is known to be first order, and the rate at which it occurs per unit area of the surface may be expressed as
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Fundamentals of Heat and Mass Transfer
- Example(2): Double effect evaporator is used for concentrating a certain caustic soda solution 10000kg/hr from 9wt% to 47wt%. The feed at 30°C enters the first evaporator. Backward arrangement evaporators are used. steam is available at 167.7°C and the vapor space in the second effect is 14.6Kpa. The overall heat transfer coefficients of the two effects are 8380 and 6285kcal/ W.CH ork -conce -SOLFFF and-ans.. 112.1 а DiD 3 respectively and the specific heat capacity of all caustic soda solution 3.771 KJ/Kg. °C, determine the heat transfer area of each effect معدلة 5:48 م Oarrow_forwardgive me solution math not explinarrow_forwardgive me solution math not explinarrow_forward
- use Q Strips of material 10 mm thick are dried under constant drying conditions from 28 to 13 per cent moisture in 25 ks. The equilibrium moisture content is 7 per cent. The relation between E, the ratio of the final free moisture content at time t to the initial free moisture content, and the parameter J is given by: E 1 0.64 0.49 0.38 0.295 0.22 0.14 J 0 0.1 0.2 0.3 0.5 0.6 العنوان 0.7 It may be noted that J = kt/12, where, k = constant, t = time (ks) 1 = thickness/2 of the sheet of material (mm) a. Based on the given data, plot a graph of E against J b. Determine the time taken to dry 60 mm planks from 22 to 10 per cent moisture under the same conditions assuming no loss from the edges? ina östler ۲/۱arrow_forward14.25.2.5 kg/s of a solution at 288 K containing 10 per cent of dissolved solids is fed to a forward-feed double-effect evaporator, operating at 14 kN/m² in the last effect. If the product is to consist of a liquid containing 50 per cent by mass of dissolved solids and dry saturated steam is fed to the steam coils, what PROBLEMS 1179 should be the pressure of the steam? The surface in each effect is 50 m² and the coefficients for heat transfer in the first and second effects are 2.8 and 1.7 kW/ m² K, respectively. It may be assumed that the concentrated solution exhibits a boiling-point rise of 5 deg K, that the latent heat has a constant value of 2260 kJ/kg and that the specific heat capacity of the liquid stream is constant at 3.75 kJ/kg K Oarrow_forward: +0 العنوان use only 5) A 100 kg batch of granular solids containing 30% moisture is to be dried in a tray drier to 15.5% by passing a current of air at 350 K tangentially across its surface at a velocity of 1.8 m/s. If the constant rate of drying under these conditions is 0.7 g/s m2 and the critical moisture content is 15%, calculate the approximate drying time. Assume the drying surface to be 0.03 m2 /kg dry mass. мониarrow_forward
- give me solution math not explinarrow_forward۲/۱ : +0 العنوان seoni 4) 1 Mg (dry weight) of a non-porous solid is dried under constant drying conditions with an air velocity of 0.75 m/s parallel to the drying surface. The area of drying surface is 55 m2 If initial rate of drying is 0.3 g/m2 s, how long it will take to dry a material from 0.15 to 0.025 kg water/kg dry solid? The critical moisture content is 0.125 and the equilibrium moisture is negligible. The falling rate of drying is linear in moisture content. If air velocity increases to 4 m/s, what will be the anticipated saving in drying time? 0 ostherarrow_forward14.23. A double-effect forward-feed evaporator is required to give a product consisting of 30 per cent crystals and a mother liquor containing 40 per cent by mass of dissolved solids. Heat transfer coefficients are 2.8 and 1.7 kW/m² K in the first and second effects respectively. Dry saturated steam is supplied at 375 kN/m² and the condenser operates at 13.5 kN/ m². (a) What area of heating surface is required in each effect assuming the effects are identical, if the feed rate is 0.6 kg/s of liquor, containing 20 per cent by mass of dissolved solids, and the feed temperature is 313 K? (b) What is the pressure above the boiling liquid in the first effect? The specific heat capacity may be taken as constant at 4.18 kJ/kg K. and the effects of boiling-point rise and of hydrostatic head may be neglected. O Oarrow_forward
- 5) A 100 kg batch of granular solids containing 30% moisture is to be dried in a tray drier to 15.5% by passing a current of air at 350 K tangentially across its surface at a velocity of 1.8 m/s. If the constant rate of drying under these conditions is 0.7 g/s m2 and the critical moisture content is 15%, calculate the approximate drying time. Assume the drying surface to be 0.03 m2 /kg dry mass. Oarrow_forwardSolve for v and Iarrow_forwardG = 0.350MPa, P = 900N, a=20mm, b=50mm, c=80mmarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





