
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.5, Problem 8P
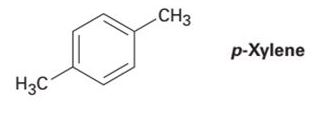
How many peaks would you expect in the 1H NMR spectrum of 1, 4-dimethyl- benzene (para-xylene, or p-xylene)? What ratio of peak areas would you expect on integration of the spectrum? Refer to Table 13-3 for approximate chemical shifts, and sketch what the spectrum would look like. (Remember from
Section 2-4
that
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Predict the major organic product(s) of the following reactions. Indicate which of the following mechanisms is in operation: SN1, SN2, E1, or E2.
(c)
(4pts)
Mechanism:
heat
(E1)
CH3OH
+
1.5pts each
_E1 _ (1pt)
Br
CH3OH
(d)
(4pts)
Mechanism:
SN1
(1pt)
(e)
(3pts)
1111 I
H
10
Ill!!
H
LDA
THF (solvent)
Mechanism: E2
(1pt)
NC
(f)
Bri!!!!!
CH3
NaCN
(3pts)
acetone
Mechanism: SN2
(1pt)
(SN1)
-OCH3
OCH3
1.5pts each
2pts for either product
1pt if incorrect
stereochemistry
H
Br
(g)
“,、
(3pts)
H
CH3OH
+21
Mechanism:
SN2
(1pt)
H
CH3
2pts
1pt if incorrect
stereochemistry
H
2pts
1pt if incorrect
stereochemistry
A mixture of butyl acrylate and 4'-chloropropiophenone has been taken for proton NMR analysis. Based on this proton NMR, determine the relative percentage of each compound in the mixture
Chapter 13 Solutions
Organic Chemistry
Ch. 13.1 - Prob. 1PCh. 13.1 - Prob. 2PCh. 13.2 - Prob. 3PCh. 13.3 - The following 1H NMR peaks were recorded on a...Ch. 13.3 - When the 1Η NMR spectrum of acetone, CH3COCH3, is...Ch. 13.4 - Each of the following compounds has a single 1H...Ch. 13.4 - Identify the different types of protons in the...Ch. 13.5 - How many peaks would you expect in the 1H NMR...Ch. 13.6 - Predict the splitting patterns you would expect...Ch. 13.6 - Draw structures for compounds that meet the...
Ch. 13.6 - The integrated 1H NMR spectrum of a compound of...Ch. 13.7 - Identify the indicated sets of protons as...Ch. 13.7 - How many kinds of electronically nonequivalent...Ch. 13.7 - How many absorptions would you expect (S)-malate,...Ch. 13.8 - 3-Bromo-1-phenyl-1-propene shows a complex NMR...Ch. 13.9 - How could you use 1H NMR to determine the...Ch. 13.11 - Prob. 17PCh. 13.11 - Propose structures for compounds that fit the...Ch. 13.11 - Prob. 19PCh. 13.12 - Prob. 20PCh. 13.12 - Prob. 21PCh. 13.12 - Prob. 22PCh. 13.13 - Prob. 23PCh. 13.SE - Into how many peaks would you expect the 1H NMR...Ch. 13.SE - How many absorptions would you expect the...Ch. 13.SE - Sketch what you might expect the 1H and 13C NMR...Ch. 13.SE - How many electronically nonequivalent kinds of...Ch. 13.SE - Identify the indicated protons in the following...Ch. 13.SE - Prob. 29APCh. 13.SE - Prob. 30APCh. 13.SE - When measured on a spectrometer operating at 200...Ch. 13.SE - Prob. 32APCh. 13.SE - Prob. 33APCh. 13.SE - How many types of nonequivalent protons are...Ch. 13.SE - The following compounds all show a single line in...Ch. 13.SE - Prob. 36APCh. 13.SE - Propose structures for compounds with the...Ch. 13.SE - Predict the splitting pattern for each kind of...Ch. 13.SE - Predict the splitting pattern for each kind of...Ch. 13.SE - Identify the indicated sets of protons as...Ch. 13.SE - Identify the indicated sets of protons as...Ch. 13.SE - The acid-catalyzed dehydration of...Ch. 13.SE - How could you use 1H NMR to distinguish between...Ch. 13.SE - Propose structures for compounds that fit the...Ch. 13.SE - Propose structures for the two compounds whose 1H...Ch. 13.SE - Prob. 46APCh. 13.SE - How many absorptions would you expect to observe...Ch. 13.SE - Prob. 48APCh. 13.SE - How could you use 1H and 13C NMR to help...Ch. 13.SE - How could you use 1H NMR, 13C NMR, and IR...Ch. 13.SE - Assign as many resonances as you can to specific...Ch. 13.SE - Assume that you have a compound with the formula...Ch. 13.SE - The compound whose 1H NMR spectrum is shown has...Ch. 13.SE - The compound whose 1H NMR spectrum is shown has...Ch. 13.SE - Propose structures for compounds that fit the...Ch. 13.SE - Long-range coupling between protons more than two...Ch. 13.SE - The 1H and 13C NMR spectra of compound A, C8H9Br,...Ch. 13.SE - Propose structures for the three compounds whose...Ch. 13.SE - The mass spectrum and 13C NMR spectrum of a...Ch. 13.SE - Compound A, a hydrocarbon with M+=96 in its mass...Ch. 13.SE - Propose a structure for compound C, which has...Ch. 13.SE - Prob. 62GPCh. 13.SE - Propose a structure for compound E, C7H12O2, which...Ch. 13.SE - Compound F, a hydrocarbon with M+=96 in its mass...Ch. 13.SE - 3-Methyl-2-butanol has five signals in its 13C NMR...Ch. 13.SE - A 13C NMR spectrum of commercially available...Ch. 13.SE - Carboxylic acids (RCO2H) react with alcohols (ROH)...Ch. 13.SE - Prob. 68GPCh. 13.SE - The proton NMR spectrum is shown for a compound...Ch. 13.SE - The proton NMR spectrum of a compound with the...Ch. 13.SE - The proton NMR spectrum is shown for a compound...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q5: Label each chiral carbon in the following molecules as R or S. Make sure the stereocenter to which each of your R/S assignments belong is perfectly clear to the grader. (8pts) R OCH 3 CI H S 2pts for each R/S HO R H !!! I OH CI HN CI R Harrow_forwardCalculate the proton and carbon chemical shifts for this structurearrow_forwardA. B. b. Now consider the two bicyclic molecules A. and B. Note that A. is a dianion and B. is a neutral molecule. One of these molecules is a highly reactive compound first characterized in frozen noble gas matrices, that self-reacts rapidly at temperatures above liquid nitrogen temperature. The other compound was isolated at room temperature in the early 1960s, and is a stable ligand used in organometallic chemistry. Which molecule is the more stable molecule, and why?arrow_forward
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY