
ORGANIC CHEMISTRY
8th Edition
ISBN: 9781323815427
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 13.23, Problem 38P
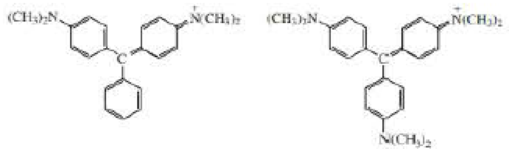
a. At pH = 7 one of the ions shown here is purple and the other is blue. Which is which? (Hint: refer to the color spectrum in figure 13.8 on page 584.)
b. What would be the difference in the colors or the compounds at pH = 3?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
[8]
1. You are taking samples at a contaminated mine site from a carbonate aquifer to determine the concentration of dissolved lead in the sample. To preserve the sample, you acidify it to pH 5. How much HCl, in equivalents/L, must be added to this sample to make it pH 5 if the total carbonate concentration is 102 M and the initial pH is 10? Note that the total carbonate is the sum of all of the carbonate species ([H2CO3]+[HCO3] + [CO2]) present in the sample. What are the acid-base pairs responsible for buffering in this sample?
Which one is more acidic and why?
Chapter 13 Solutions
ORGANIC CHEMISTRY
Ch. 13.1 - Which of the following fragments produced in a...Ch. 13.2 - What distinguishes the mass spectrum of...Ch. 13.2 - What is the most likely m/z value for the base...Ch. 13.3 - Prob. 5PCh. 13.3 - a. Suggest possible molecular formulas for a...Ch. 13.3 - If a compound has a molecular ion with an...Ch. 13.3 - Identify the hydrocarbon that has a molecular ion...Ch. 13.4 - Predict the relative intensities of the molecular...Ch. 13.5 - Which molecular formula has an exact molecular...Ch. 13.5 - Prob. 11P
Ch. 13.6 - Sketch the mass spectrum expected for...Ch. 13.6 - The mass spectra of 1-methoxybutane,...Ch. 13.6 - Primary alcohols have a strong peak at m/z = 31....Ch. 13.6 - Identify the ketones responsible for the mass...Ch. 13.6 - Prob. 16PCh. 13.6 - Using curved arrows, show the principal fragments...Ch. 13.6 - The reaction of (Z)-2-pentene with water and a...Ch. 13.9 - a. Which is higher in energy: electromagnetic...Ch. 13.9 - Prob. 20PCh. 13.13 - Prob. 21PCh. 13.14 - Which occur at a larger wavenumber: a. the C O...Ch. 13.14 - Prob. 23PCh. 13.14 - Prob. 24PCh. 13.14 - Rank the following compounds from highest...Ch. 13.14 - Which shows an O H stretch at a larger...Ch. 13.16 - Prob. 27PCh. 13.16 - a. An oxygen-containing compound shows an...Ch. 13.16 - Prob. 29PCh. 13.16 - For each of the following pair of compounds, name...Ch. 13.17 - Which of the following compounds has a vibration...Ch. 13.17 - Prob. 32PCh. 13.18 - A compound with molecular formula C4H6O gives the...Ch. 13.20 - Prob. 34PCh. 13.20 - Prob. 35PCh. 13.21 - Predict the max of the following compound:Ch. 13.21 - Prob. 37PCh. 13.23 - a. At pH = 7 one of the ions shown here is purple...Ch. 13.23 - Prob. 39PCh. 13.23 - Prob. 40PCh. 13 - In the mass spectrum of the following compounds,...Ch. 13 - Prob. 42PCh. 13 - Draw structures for a saturated hydrocarbon that...Ch. 13 - Rank the following compounds in order of...Ch. 13 - For each of the following pairs of compounds,...Ch. 13 - a. How could you use IR spectroscopy to determine...Ch. 13 - Assuming that the force constant is approximately...Ch. 13 - Norlutin and Enovid are ketones that suppress so...Ch. 13 - In the following boxes, list the types of bonds...Ch. 13 - A mass spectrum shows significant peaks at m/z. =...Ch. 13 - Prob. 51PCh. 13 - Prob. 52PCh. 13 - Prob. 53PCh. 13 - The IR spectrum of a compound with molecular...Ch. 13 - Rank the following compounds from highest...Ch. 13 - Rank the following compounds from highest...Ch. 13 - What peaks in their mass spectra can be used to...Ch. 13 - Prob. 58PCh. 13 - Which one of the following five compounds produced...Ch. 13 - Prob. 60PCh. 13 - Each of the IR spectra shown below is accompanied...Ch. 13 - Prob. 62PCh. 13 - Prob. 63PCh. 13 - How can IR spectroscopy distinguish between...Ch. 13 - Prob. 65PCh. 13 - Prob. 66PCh. 13 - Give approximate wavenumbers for the major...Ch. 13 - Prob. 68PCh. 13 - Which one of the following live compounds produced...Ch. 13 - Phenolphthalein is an acid-base indicator. In...Ch. 13 - Prob. 71PCh. 13 - How can you use UV spectroscopy to distinguish...Ch. 13 - Prob. 73PCh. 13 - The IR and mass spectra for three different...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- We have seen an introductory definition of an acid: An acid is a compound that reacts with water and increases the amount of hydronium ion present. In the Chapter on acids and bases, we saw two more definitions of acids: a compound that donates a proton (a hydrogen ion, H+) to another compound is called a Bronsted-Lowry acid, and a Lewis acid is any species that can accept a pair of electrons. Explain why the introductory definition is a macroscopic definition, while the Bronsted-Lomy definition and the Lewis definition are microscopic definitions.arrow_forwardWhat are the equilibrium concentration of H3O+, CN and HCN in a 0.025 M solution of HCN? What is the pH of the solution?arrow_forward6. Both CO3 and HCO3 respond to the usual carbonate ion test. Give specific procedures by which you could distinguish between these ions in an unknown. (Hint: Consider the pH of the solution.) 2-arrow_forward
- 1. Ammonia in solution. a. Write the net ionic equation that shows the reaction of 1.0 M ammonia, NH,, when dissolved in water. b. At room temperature, the K, of the above solution is 1.8 x 105. If the initial concentration of NH, is 1.0 M, quantitatively define the pH of the above solution. (Show calculations using an I.C.E table and use the quadratic equation B c. What is the reaction that occurs when a solution of ammonia is added to a solution containing Fe³* ions d. Propose a chemical compound that can be used to dissolve the precipitate formed in part 1.c. Support your answer with a proper chemical equationarrow_forwardIn the laboratory, a general chemistry student measured the pH of a 0.386 M aqueous solution of nitrous acid to be 1.865. Use the information she obtained to determine the Ka for this acid. Ka(experiment) = In the laboratory, a general chemistry student measured the pH of a 0.431 M aqueous solution of hydrofluoric acid to be 1.741. Use the information she obtained to determine the Ka for this acid. Ka(experiment) =arrow_forward12. Compare the surface activity of propionic and butyric acids in aqueous solutions in this concentration range: C, mol/L a, mN/mol Propionic acid Butyric acid 0.0312 0.0625 Is the Traube's Rule complied? 69.5 65.8 67.7 60.4arrow_forward
- You are given a 0.250 M NO21 solution. a. Write the balanced chemical reaction of NO21 with water, including phase labels and correct charges. b. Using an ICE table, calculate the pH of this weak base solution. The Kb of NO21 is 2.16 · 10-11.arrow_forwardAssume each salt below is dissolved in water to make a 1M solution. Chose if that solution is acidic, basic, neutral, or you would need more information to make that determination. • (CH3)3NHF [ Select] • NaHSO4 Acidic • Na2SO4 [Select] • Ca(CO3)2 [Ca(CO3)2] • C5H5NHBr Acidicarrow_forwardA substance has a pH of 5.3. Their [H*] isarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
Acid-base Theories and Conjugate Acid-base Pairs; Author: Mindset;https://www.youtube.com/watch?v=hQLWYmAFo3E;License: Standard YouTube License, CC-BY
COMPLEXOMETRIC TITRATION; Author: Pikai Pharmacy;https://www.youtube.com/watch?v=EQxvY6a42Dw;License: Standard Youtube License