
Concept explainers
Lakes freeze from top down we all know that ice cubes float in a glass of water. Why? Virtually every substance contracts when it solidifies—the solid is denser than the liquid. If this happened to water. Ice cubes would sink to the bottom of a glass, and ice sheets would sink to the bottom of a lake. Fortunately, this doesn't happen. Liquid water expands by
But this is not the only special thing about water. While the density of most substances increases when they are cooled, water density shows a very peculiar temperature dependence (see Figure 13.17). As the temperature decreases, water density increases, but only until
Note that if water were most dense at the freezing point, then in the winter the very cold water at the surface of lakes would sink in this case the lake would freeze from the bottom up, and an life in it would be destroyed.
The expansion of water when it freezes has another important environmental benefit the so-called freeze-thaw effect on sedimentary rocks. Water is absorbed into cracks in these rocks and men freezes in cold weather. The solid ice expands and cracks me rock like a wood-cutter splitting logs. This continual process of liquid water absorption, freezing, and cracking releases mineral and nitrogen deposits into me soil and can eventual break the rock down into soil.
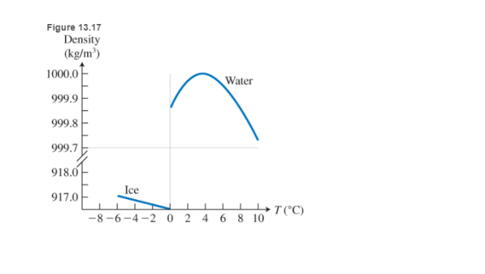
When is water densest?
a. When liquid at
b. When solid ice at
c. When liquid at
d. Water density is always
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
College Physics
Additional Science Textbook Solutions
Campbell Biology in Focus (2nd Edition)
Applications and Investigations in Earth Science (9th Edition)
College Physics: A Strategic Approach (3rd Edition)
Anatomy & Physiology (6th Edition)
Introductory Chemistry (6th Edition)
Campbell Biology (11th Edition)
- Question B3 Consider the following FLRW spacetime: t2 ds² = -dt² + (dx² + dy²+ dz²), t2 where t is a constant. a) State whether this universe is spatially open, closed or flat. [2 marks] b) Determine the Hubble factor H(t), and represent it in a (roughly drawn) plot as a function of time t, starting at t = 0. [3 marks] c) Taking galaxy A to be located at (x, y, z) = (0,0,0), determine the proper distance to galaxy B located at (x, y, z) = (L, 0, 0). Determine the recessional velocity of galaxy B with respect to galaxy A. d) The Friedmann equations are 2 k 8πG а 4πG + a² (p+3p). 3 a 3 [5 marks] Use these equations to determine the energy density p(t) and the pressure p(t) for the FLRW spacetime specified at the top of the page. [5 marks] e) Given the result of question B3.d, state whether the FLRW universe in question is (i) radiation-dominated, (ii) matter-dominated, (iii) cosmological-constant-dominated, or (iv) none of the previous. Justify your answer. f) [5 marks] A conformally…arrow_forwardSECTION B Answer ONLY TWO questions in Section B [Expect to use one single-sided A4 page for each Section-B sub question.] Question B1 Consider the line element where w is a constant. ds²=-dt²+e2wt dx², a) Determine the components of the metric and of the inverse metric. [2 marks] b) Determine the Christoffel symbols. [See the Appendix of this document.] [10 marks] c) Write down the geodesic equations. [5 marks] d) Show that e2wt it is a constant of geodesic motion. [4 marks] e) Solve the geodesic equations for null geodesics. [4 marks]arrow_forwardPage 2 SECTION A Answer ALL questions in Section A [Expect to use one single-sided A4 page for each Section-A sub question.] Question A1 SPA6308 (2024) Consider Minkowski spacetime in Cartesian coordinates th = (t, x, y, z), such that ds² = dt² + dx² + dy² + dz². (a) Consider the vector with components V" = (1,-1,0,0). Determine V and V. V. (b) Consider now the coordinate system x' (u, v, y, z) such that u =t-x, v=t+x. [2 marks] Write down the line element, the metric, the Christoffel symbols and the Riemann curvature tensor in the new coordinates. [See the Appendix of this document.] [5 marks] (c) Determine V", that is, write the object in question A1.a in the coordinate system x'. Verify explicitly that V. V is invariant under the coordinate transformation. Question A2 [5 marks] Suppose that A, is a covector field, and consider the object Fv=AAμ. (a) Show explicitly that F is a tensor, that is, show that it transforms appropriately under a coordinate transformation. [5 marks] (b)…arrow_forward
- No chatgpt pls will upvote Iarrow_forwardHow would partial obstruction of an air intake port of an air-entrainment mask effect FiO2 and flow?arrow_forward14 Z In figure, a closed surface with q=b= 0.4m/ C = 0.6m if the left edge of the closed surface at position X=a, if E is non-uniform and is given by € = (3 + 2x²) ŷ N/C, calculate the (3+2x²) net electric flux leaving the closed surface.arrow_forward
- No chatgpt pls will upvotearrow_forwardsuggest a reason ultrasound cleaning is better than cleaning by hand?arrow_forwardCheckpoint 4 The figure shows four orientations of an electric di- pole in an external electric field. Rank the orienta- tions according to (a) the magnitude of the torque on the dipole and (b) the potential energy of the di- pole, greatest first. (1) (2) E (4)arrow_forward

 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill





