Expression for Kf' is as follows:
Kf'=(αY4−)(Kf) (1)
Here,
Kf' is conditional rate constant.
Kf is rate constant.
αY4− is fraction of free EDTA in form of Y4−.
Substitute 7.41×1013 for Kf and 4.2×10−3 for αY4− in equation (1).
Kf'=(4.2×10−3)(7.41×1013)=31.1×1010
At 0 mL of EDTA solution,
Expression to calculate pMn2+ is as follows:
pMn2+=−log[Mn2+] (2)
Substitute 0.0200 M for [Mn2+] in equation (2).
pMn2+=−log(0.0200 M)=1.698≈1.70
When 0 mL of EDTA is added to Mn2+ solution, pMn2+ is 1.70.
At 20.0 mL of EDTA solution,
Expression to calculate total volume of solution is as follows:
Total volume of solution=(Volume of Mn2++Volume of EDTA) (3)
Substitute 25.0 mL for volume of Mn2+ and 20.0 mL for volume of EDTA in equation (3).
Total volume of solution=(25.0+20.0) mL=45 mL
Since EDTA forms strong metal complexes in the ratio of 1:1, moles of EDTA will be equivalent to moles of metal reacted.
Expression to calculate excess concentration of Mn2+ is as follows:
Excess [Mn2+]=(([Mn2+])(Volume of Mn2+)−([EDTA])(Volume of EDTA)Total volume of solution) (4)
Substitute 25.0 mL for volume of Mn2+, 0.0200 M for [Mn2+], 20.0 mL for volume of EDTA, 0.0100 M for [EDTA] and 45 mL for total volume of solution in equation (4).
Excess [Mn2+]=((0.0200 M)(25.0 mL)−(0.0100 M)(20.0 mL)45 mL)=0.00667 M
Substitute 0.00667 M for [Mn2+] in equation (2).
pMn2+=−log(0.00667 M)=2.18
At 40.0 mL of EDTA solution,
Substitute 25.0 mL for volume of Mn2+ and 40.0 mL for volume of EDTA in equation (3).
Total volume of solution=(25.0+40.0) mL=65 mL
Substitute 25.0 mL for volume of Mn2+, 0.0200 M for [Mn2+], 40.0 mL for volume of EDTA, 0.0100 M for [EDTA] and 65 mL for total volume of solution in equation (4).
Excess [Mn2+]=((0.0200 M)(25.0 mL)−(0.0100 M)(40.0 mL)65 mL)=0.00154 M
Substitute 0.00154 M for [Mn2+] in equation (2).
pMn2+=−log(0.00154 M)=2.81
At 49.0 mL of EDTA solution,
Substitute 25.0 mL for volume of Mn2+ and 49.0 mL for volume of EDTA in equation (3).
Total volume of solution=(25.0+49.0) mL=74 mL
Substitute 25.0 mL for volume of Mn2+, 0.0200 M for [Mn2+], 49.0 mL for volume of EDTA, 0.0100 M for [EDTA] and 74 mL for total volume of solution in equation (4).
Excess [Mn2+]=((0.0200 M)(25.0 mL)−(0.0100 M)(49.0 mL)74 mL)=0.000135 M
Substitute 0.000135 M for [Mn2+] in equation (2).
pMn2+=−log(0.000135 M)=3.87
At 49.9 mL of EDTA solution,
Substitute 25.0 mL for volume of Mn2+ and 49.9 mL for volume of EDTA in equation (3).
Total volume of solution=(25.0+49.9) mL=74.9 mL
Substitute 25.0 mL for volume of Mn2+, 0.0200 M for [Mn2+], 49.9 mL for volume of EDTA, 0.0100 M for [EDTA] and 74.9 mL for total volume of solution in equation (4).
Excess [Mn2+]=((0.0200 M)(25.0 mL)−(0.0100 M)(49.9 mL)74.9 mL)=0.0000013 M
Substitute 0.0000013 M for [Mn2+] in equation (2).
pMn2+=−log(0.0000013 M)=5.89
At 50.0 mL of EDTA solution,
Formula to calculate molarity of solution is as follows:
Molarity of solution(M)=Moles of soluteVolume (L) of solution (5)
Rearrange equation (5) for moles of solute.
Moles of solute=[(Molarity of solution)(Volume of solution)] (6)
Substitute 0.0200 M for molarity and 25.0 mL for volume of solution in equation (6) to calculate millimoles of Mn2+.
Millimoles of Mn2+=(0.0200 M)(25.0 mL)=0.5 mmol
Substitute 0.0100 M for molarity and 50.0 mL for volume of solution in equation (6) to calculate millimoles of EDTA.
Millimoles of EDTA=(0.0100 M)(50.0 mL)=0.5 mmol
Substitute 25.0 mL for volume of Mn2+ and 50.0 mL for volume of EDTA in equation (3).
Total volume of solution=(25.0+50.0) mL=75 mL
Chemical reaction occurs as follows:
Mn2++Y2−↔MnY2−
Concentration of MnY2− at equivalence point is calculated as follows:
[MnY2−]=0.5 mmol75 mL=0.0067 M
Consider change in concentrations of ionic species to be negligible and amount of Mn2+ and EDTA to be x.
Expression to calculate Kf' at equivalence point is as follows:
Kf'=[MnY2−][Mn2+][EDTA] (7)
Substitute 0.0067 M for [MnY2−], x for [Mn2+], x for [EDTA] and 31.1×1010 for Kf' in equation (7).
31.1×1010=0.0067 M(x)(x)
Solve for x,
x=1.27×10−7 M
Substitute 1.27×10−7 M for [Mn2+] in equation (2).
pMn2+=−log(1.27×10−7 M)=6.89
At 50.0 mL of EDTA solution,
Substitute 25.0 mL for volume of Mn2+ and 50.1 mL for volume of EDTA in equation (3).
Total volume of solution=(25.0+50.1) mL=75.1 mL
Chemical reaction occurs as follows:
Mn2++Y2−↔MnY2−
Concentration of MnY2− at equivalence point is calculated as follows:
[MnY2−]=0.5 mmol75.1 mL=0.00066 M
Concentration of excess EDTA is calculated as follows:
Excess [EDTA]=(0.0100 M)(0.1 mL)75.1 mL=1.33×10−5 M
Rearrange equation (7) for [Mn2+].
[Mn2+]=[MnY2−]Kf'[EDTA] (8)
Substitute 0.00066 M for [MnY2−], 1.33×10−5 M for [EDTA] and 31.1×1010 for Kf' in equation (8).
[Mn2+]=0.00066 M(31.1×1010)(1.33×10−5 M)=1.60×10−10 M
Substitute 1.60×10−10 M for [Mn2+] in equation (2).
pMn2+=−log(1.60×10−10 M)=9.80
At 55.0 mL of EDTA solution,
Substitute 25.0 mL for volume of Mn2+ and 55.0 mL for volume of EDTA in equation (3).
Total volume of solution=(25.0+55.1) mL=80 mL
Chemical reaction occurs as follows:
Mn2++Y2−↔MnY2−
Concentration of MnY2− at equivalence point is calculated as follows:
[MnY2−]=0.5 mmol80 mL=6.25×10−3 M
Concentration of excess EDTA is calculated as follows:
Excess [EDTA]=(0.0100 M)(0.1 mL)80 mL=6.25×10−4 M
Substitute 6.25×10−3 M for [MnY2−], 6.25×10−4 M for [EDTA] and 31.1×1010 for Kf' in equation (8).
[Mn2+]=6.25×10−3 M(31.1×1010)(6.25×10−4 M)=3.215×10−11 M
Substitute 3.215×10−11 M for [Mn2+] in equation (2).
pMn2+=−log(3.215×10−11 M)=10.49
At 60.0 mL of EDTA solution,
Substitute 25.0 mL for volume of Mn2+ and 60.0 mL for volume of EDTA in equation (3).
Total volume of solution=(25.0+60.1) mL=85 mL
Chemical reaction occurs as follows:
Mn2++Y2−↔MnY2−
Concentration of MnY2− at equivalence point is calculated as follows:
[MnY2−]=0.5 mmol85 mL=5.88×10−3 M
Concentration of excess EDTA is calculated as follows:
Excess [EDTA]=(0.0100 M)(0.1 mL)85 mL=1.18×10−3 M
Substitute 5.88×10−3 M for [MnY2−], 1.18×10−3 M for [EDTA] and 31.1×1010 for Kf' in equation (8).
[Mn2+]=5.88×10−3 M(31.1×1010)(1.18×10−3 M)=1.602×10−11 M
Substitute 1.602×10−11 M for [Mn2+] in equation (2).
pMn2+=−log(1.602×10−11 M)=10.80
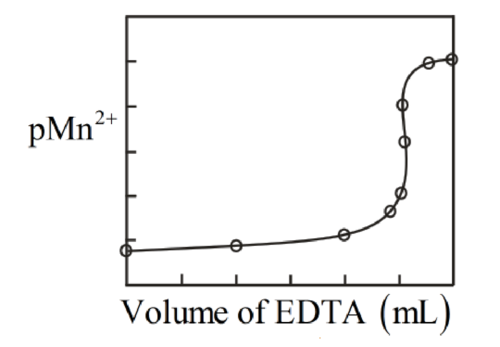
Titration curve for the same is drawn as follows:


 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY




