
Concept explainers
(a)
Interpretation:
The starting material to prepare the following
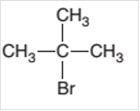
Concept Introduction:
A
In a chemical reaction; the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactant and products must be separated by an arrow.
Halogenation reaction is a substitution reaction in which one of the H atom of reactant hydrocarbon is substituted with halogen atom to form respective alkyl halide.
(b)
Interpretation:
The starting material to prepare the following alkyl halide by a halogenation reaction should be determined:
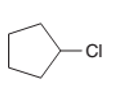
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction; the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactant and products must be separated by an arrow.
Halogenation reaction is a substitution reaction in which one of the H atom of reactant hydrocarbon is substituted with halogen atom to form respective alkyl halide.
(c)
Interpretation:
The starting material to prepare the following alkyl halide by a halogenation reaction should be determined:
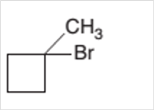
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction; the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactant and products must be separated by an arrow.
Halogenation reaction is a substitution reaction in which one of the H atom of reactant hydrocarbon is substituted with halogen atom to form respective alkyl halide.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
General, Organic, & Biological Chemistry
- Which alkane most readily undergoes thermal decomposition? Note that C-H bonds are usually stronger than C-C bonds. O ethane O dimethylpropane O propane O methylpropanearrow_forwardWhat functional group is formed after tautomerization during hydroboration of an alkyne? Provide an example that includes the reactant, reagent and product.arrow_forwardWhat kinds of reagents add to the weak, electron-rich π bond of alkenes?arrow_forward
- How to name an epoxide as an epoxyalkane ?arrow_forwardTrue or False Considering that two carbon chains have equal number of carbons, but one has Fluorine and the other has Iodine, the one with iodine will have a higher boiling point. Mild oxidation of alkenes results to similar product as that of nucleophilic addition of water to aldehydes.arrow_forwardName the following alkyne by an acceptable system of nomenclature:arrow_forward
- What is the systematic name of the alkyl halide product?arrow_forwardHow many monochloro substitution products are produced when the alkane below is chlorinated? Consider both constitutional isomers and stereoisomers. d The number of monochloro substitution products isarrow_forwardHow many alkene products are possible in the following reaction? CI CH3 NaOEt {"CH3 Harrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


