
Chemistry: Atoms First
3rd Edition
ISBN: 9781259638138
Author: Julia Burdge, Jason Overby Professor
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 12.4, Problem 3PPC
Interpretation Introduction
Interpretation:
The diagram of an edge-centered unit cell of an ionic compound in which ions are in
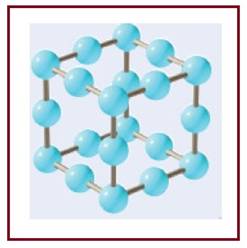
Figure 1
The number of ions per edge-centered cubic unit cell has to be determined.
Concept Introduction:
A cube has
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A certain half-reaction has a standard reduction potential Ered +1.26 V. An engineer proposes using this half-reaction at the anode of a galvanic cell that
must provide at least 1.10 V of electrical power. The cell will operate under standard conditions.
Note for advanced students: assume the engineer requires this half-reaction to happen at the anode of the cell.
Is there a minimum standard reduction
potential that the half-reaction used at
the cathode of this cell can have?
If so, check the "yes" box and calculate
the minimum. Round your answer to 2
decimal places. If there is no lower
limit, check the "no" box..
Is there a maximum standard reduction
potential that the half-reaction used at
the cathode of this cell can have?
If so, check the "yes" box and calculate
the maximum. Round your answer to 2
decimal places. If there is no upper
limit, check the "no" box.
yes, there is a minimum.
1
red
Πν
no minimum
Oyes, there is a maximum.
0
E
red
Dv
By using the information in the ALEKS…
In statistical thermodynamics, check the
hcv
following equality: ß Aɛ =
KT
Please correct answer and don't used hand raiting
Chapter 12 Solutions
Chemistry: Atoms First
Ch. 12.2 - Prob. 12.1WECh. 12.2 - Prob. 1PPACh. 12.2 - Prob. 1PPBCh. 12.2 - The diagram on the left depicts a system at room...Ch. 12.2 - Prob. 12.2.1SRCh. 12.2 - Given the following information for C6F6,...Ch. 12.2 - Prob. 12.2.3SRCh. 12.2 - Using the result from question 12.2.3 and another...Ch. 12.3 - Prob. 12.2WECh. 12.3 - When silver crystallizes, it forms face-centered...
Ch. 12.3 - The density of sodium metal is 0.971 g/cm3 and the...Ch. 12.3 - Nickel has a face-centered cubic unit cell with an...Ch. 12.3 - A metal crystallizes in a body-centered cubic unit...Ch. 12.4 - How many of each ion are contained within a unit...Ch. 12.4 - Referring to Figure 12.23, determine how many of...Ch. 12.4 - Referring to Figure 12.23, determine how many of...Ch. 12.4 - Prob. 3PPCCh. 12.4 - The edge length of the NaCl unit cell is 564 pm....Ch. 12.4 - Prob. 4PPACh. 12.4 - NiO also adopts the face-centered cubic...Ch. 12.4 - The metal iridium (Ir) crystallizes with a...Ch. 12.4 - Prob. 5PPACh. 12.4 - Copper crystallizes in a face-centered cubic...Ch. 12.4 - Given that the diameter and average mass of a...Ch. 12.5 - (a) Calculate the amount of heat deposited oil the...Ch. 12.5 - Calculate the amount of energy (in kilojoules)...Ch. 12.5 - Determine the final state and temperature of 100 g...Ch. 12.5 - Prob. 6PPCCh. 12.5 - How much energy (in kilojoules) is required to...Ch. 12.5 - Prob. 12.5.2SRCh. 12.6 - Using the following phase diagram, (a) determine...Ch. 12.6 - Use the following phase diagram to (a) determine...Ch. 12.6 - Prob. 7PPBCh. 12.6 - Prob. 7PPCCh. 12.6 - Prob. 12.6.1SRCh. 12.6 - Prob. 12.6.2SRCh. 12 - Prob. 12.1KSPCh. 12 - Prob. 12.2KSPCh. 12 - Prob. 12.3KSPCh. 12 - Prob. 12.4KSPCh. 12 - Explain why liquids, unlike gases, are virtually...Ch. 12 - What is surface tension? What is the relationship...Ch. 12 - Prob. 12.3QPCh. 12 - Prob. 12.4QPCh. 12 - Prob. 12.5QPCh. 12 - Prob. 12.6QPCh. 12 - Prob. 12.7QPCh. 12 - Why does the viscosity of a liquid decrease with...Ch. 12 - Why is ice less dense than water?Ch. 12 - Prob. 12.10QPCh. 12 - Prob. 12.11QPCh. 12 - Prob. 12.12QPCh. 12 - Prob. 12.13QPCh. 12 - Predict which of the following liquids has greater...Ch. 12 - Prob. 12.15QPCh. 12 - Vapor pressure measurements at several different...Ch. 12 - The vapor pressure of liquid X is lower than that...Ch. 12 - Prob. 12.18QPCh. 12 - Prob. 12.19QPCh. 12 - Define the following terms: crystalline solid,...Ch. 12 - Prob. 12.21QPCh. 12 - Classify the solid states in terms of crystal...Ch. 12 - Prob. 12.23QPCh. 12 - Define X-ray diffraction. What are the typical...Ch. 12 - Prob. 12.25QPCh. 12 - What is the coordination number of each sphere in...Ch. 12 - Calculate the number of spheres that would be...Ch. 12 - Prob. 12.28QPCh. 12 - Barium metal crystallizes in a body-centered cubic...Ch. 12 - Prob. 12.30QPCh. 12 - Europium crystallizes in a body-centered cubic...Ch. 12 - Crystalline silicon has a cubic structure. The...Ch. 12 - Prob. 12.33QPCh. 12 - Prob. 12.34QPCh. 12 - Prob. 12.35QPCh. 12 - Prob. 12.36QPCh. 12 - Shown here is a zinc oxide unit cell. What is the...Ch. 12 - Describe and give examples of the following types...Ch. 12 - Prob. 12.39QPCh. 12 - A solid is hard, brittle, and electrically...Ch. 12 - A solid is soft and has a low melting point (below...Ch. 12 - Prob. 12.42QPCh. 12 - Which of the following are molecular solids and...Ch. 12 - Prob. 12.44QPCh. 12 - Prob. 12.45QPCh. 12 - What is a phase change? Name all possible changes...Ch. 12 - What is the equilibrium vapor pressure of a...Ch. 12 - Use any one of the phase changes to explain what...Ch. 12 - Define the following terms: (a) molar heat of...Ch. 12 - Prob. 12.50QPCh. 12 - What can we learn about the intermolecular forces...Ch. 12 - The greater the molar heat of vaporization of a...Ch. 12 - Prob. 12.53QPCh. 12 - A closed container of liquid pentane (bp = 36.1C)...Ch. 12 - What is critical temperature? What is the...Ch. 12 - Prob. 12.56QPCh. 12 - How do the boiling points and melting points of...Ch. 12 - Prob. 12.58QPCh. 12 - Prob. 12.59QPCh. 12 - Prob. 12.60QPCh. 12 - Which of the following phase transitions gives off...Ch. 12 - Prob. 12.62QPCh. 12 - Prob. 12.63QPCh. 12 - Calculate the amount of heat (in kilo joules)...Ch. 12 - Prob. 12.65QPCh. 12 - The molar heats of fusion and sublimation of lead...Ch. 12 - Prob. 12.67QPCh. 12 - How is the rate of evaporation of a liquid...Ch. 12 - Explain why steam at 100C causes more serious bums...Ch. 12 - The following compounds, listed with then- boiling...Ch. 12 - Prob. 12.71QPCh. 12 - Prob. 12.72QPCh. 12 - Explain how waters phase diagram differs from...Ch. 12 - The blades of ice skates are quite thin, so the...Ch. 12 - A length of wire is placed on top of a block of...Ch. 12 - Prob. 12.76QPCh. 12 - A phase diagram of water is shown. Label the...Ch. 12 - Prob. 12.78QPCh. 12 - Prob. 12.79QPCh. 12 - Prob. 12.80QPCh. 12 - Prob. 12.81QPCh. 12 - Prob. 12.82QPCh. 12 - The average distance between base pairs measured...Ch. 12 - A CO2 fire extinguisher is located on the outside...Ch. 12 - What is the vapor pressure of mercury at its...Ch. 12 - Prob. 12.86QPCh. 12 - The liquid-vapor boundary line in the phase...Ch. 12 - Prob. 12.88QPCh. 12 - Prob. 12.89QPCh. 12 - A student is given four solid samples labeled W,...Ch. 12 - Prob. 12.91QPCh. 12 - The diagram shows a kettle of boiling water....Ch. 12 - The south pole of Mars is covered with solid...Ch. 12 - The properties of gases, liquids, and solids...Ch. 12 - The standard enthalpy of formation of gaseous...Ch. 12 - Prob. 12.96QPCh. 12 - Under the same conditions of temperature and...Ch. 12 - The distance between Li+ and Cl is 257 pm in solid...Ch. 12 - Heat of hydration, that is, the heat change that...Ch. 12 - The fluorides of the second period elements and...Ch. 12 - Calculate the H for the following processes at...Ch. 12 - Prob. 12.102QPCh. 12 - Prob. 12.103QPCh. 12 - Ozone (O3) is a strong oxidizing agent that can...Ch. 12 - A sample of limestone (CaCO3) is heated in a...Ch. 12 - Carbon and silicon belong to Group 4A of the...Ch. 12 - Prob. 12.107QPCh. 12 - A 1.20-g sample of water is injected into an...Ch. 12 - What are the advantages of cooking the vegetable...Ch. 12 - A quantitative measure of how efficiently spheres...Ch. 12 - The phase diagram of helium is shown. Helium is...Ch. 12 - The phase diagram of sulfur is shown. (a) How many...Ch. 12 - Prob. 12.113QPCh. 12 - Argon crystallizes in the face-centered cubic...Ch. 12 - Given the phase diagram of carbon, answer the...Ch. 12 - Prob. 12.116QPCh. 12 - Swimming coaches sometimes suggest that a drop of...Ch. 12 - Prob. 12.118QPCh. 12 - Why do citrus growers spray their trees with water...Ch. 12 - Calcium metal crystallizes in a face-centered...Ch. 12 - A student heated a beaker of cold water (on a...Ch. 12 - The compound diclilorodifluoromethane (CCl2F2) has...Ch. 12 - Iron crystallizes in a body-centered cubic...Ch. 12 - Sketch the cooling curves of water from about 110C...Ch. 12 - Prob. 12.125QPCh. 12 - A sampleof water shows the following behavior as...Ch. 12 - A closed vessel of volume 9.6 L contains 2.0 g of...Ch. 12 - The electrical conductance of copper metal...Ch. 12 - Assuming ideal behavior, calculate the density of...Ch. 12 - Explain why drivers are advised to use motor oil...Ch. 12 - Prob. 12.131QPCh. 12 - Silicon used in computer chips must have an...Ch. 12 - Prob. 12.133QP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please correct answer and don't used hand raitingarrow_forward(11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B Bond A Bond C a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest Bond Strongest Bond b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. c. (5pts) Use principles discussed in lecture, supported by relevant structures, to succinctly explain the why your part b (i) radical is more stable than your part b(ii) radical. Written explanation can be no more than one-two succinct sentence(s)!arrow_forward. 3°C with TH 12. (10pts total) Provide the major product for each reaction depicted below. If no reaction occurs write NR. Assume heat dissipation is carefully controlled in the fluorine reaction. 3H 24 total (30) 24 21 2h • 6H total ● 8H total 34 래 Br2 hv major product will be most Substituted 12 hv Br NR I too weak of a participate in P-1 F₂ hv Statistically most favored product will be major = most subst = thermo favored hydrogen atom abstractor to LL Farrow_forward
- Five chemistry project topic that does not involve practicalarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardQ2. Consider the hydrogenation of ethylene C2H4 + H2 = C2H6 The heats of combustion and molar entropies for the three gases at 298 K are given by: C2H4 C2H6 H2 AH comb/kJ mol¹ -1395 -1550 -243 Sº / J K¹ mol-1 220.7 230.4 131.1 The average heat capacity change, ACP, for the reaction over the temperature range 298-1000 K is 10.9 J K¹ mol¹. Using these data, determine: (a) the standard enthalpy change at 800 K (b) the standard entropy change at 800 K (c) the equilibrium constant at 800 K.arrow_forward
- 13. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B Bond A Bond C a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest Bond Strongest Bond b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. c. (5pts) Use principles discussed in lecture, supported by relevant structures, to succinctly explain the why your part b (i) radical is more stable than your part b(ii) radical. Written explanation can be no more than one-two succinct sentence(s)! Googlearrow_forwardPrint Last Name, First Name Initial Statifically more chances to abstract one of these 6H 11. (10pts total) Consider the radical chlorination of 1,3-diethylcyclohexane depicted below. 4 4th total • 6H total 래 • 4H total 21 total ZH 2H Statistical H < 3° C-H weakest - product abstraction here bund leads to thermo favored a) (6pts) How many unique mono-chlorinated products can be formed and what are the structures for the thermodynamically and statistically favored products? Product 6 Number of Unique Mono-Chlorinated Products Thermodynamically Favored Product Statistically Favored Product b) (4pts) Draw the arrow pushing mechanism for the FIRST propagation step (p-1) for the formation of the thermodynamically favored product. Only draw the p-1 step. You do not need to include lone pairs of electrons. No enthalpy calculation necessary H H-Cl Waterfoxarrow_forward10. (5pts) Provide the complete arrow pushing mechanism for the chemical transformation → depicted below Use proper curved arrow notation that explicitly illustrates all bonds being broken, and all bonds formed in the transformation. Also, be sure to include all lone pairs and formal charges on all atoms involved in the flow of electrons. CH3O II HA H CH3O-H H ①arrow_forward
- Do the Lone Pairs get added bc its valence e's are a total of 6 for oxygen and that completes it or due to other reasons. How do we know the particular indication of such.arrow_forwardNGLISH b) Identify the bonds present in the molecule drawn (s) above. (break) State the function of the following equipments found in laboratory. Omka) a) Gas mask b) Fire extinguisher c) Safety glasses 4. 60cm³ of oxygen gas diffused through a porous hole in 50 seconds. How long w 80cm³ of sulphur(IV) oxide to diffuse through the same hole under the same conditions (S-32.0.0-16.0) (3 m 5. In an experiment, a piece of magnesium ribbon was cleaned with steel w clean magnesium ribbon was placed in a crucible and completely burnt in oxy cooling the product weighed 4.0g a) Explain why it is necessary to clean magnesium ribbon. Masterclass Holiday assignmen PB 2arrow_forwardHi!! Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year organic chemistry course. ****the solution must include all steps, mechanisms, and intermediate structures as required. Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!! I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning  Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=HCWwRh5CXYU;License: Standard YouTube License, CC-BY