
(a)
Interpretation:
The conversion of N-methylbenzylamine to given compound has to be explained.
Concept introduction:
The molecular formula of N-methylbutanamide is
The structure of N- methylbutanamide is as follows.
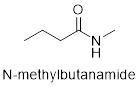
(b)
Interpretation:
The conversion of N-methylbenzylamine to given compound has to be explained.
Concept introduction:
The molecular formula of N-methylbutanamide is
The structure of N- methylbutanamide is as follows.

(c)
Interpretation:
The conversion of N-methylbenzylamine to given compound has to be explained.
Concept introduction:
The molecular formula of N-methylbutanamide is
The structure of N- methylbutanamide is as follows.

(d)
Interpretation:
The conversion of N-methylbenzylamine to given compound has to be explained.
Concept introduction:
The molecular formula of N-methylbutanamide is
The structure of N- methylbutanamide is as follows.

Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Essential Organic Chemistry (3rd Edition)
- Which of the following compounds will NOT test positive in a hydroxamic test? a. butanoic acid b. butanoyl chloride c. butyl ethanoate d. butanoic acid anhydridearrow_forwardQUESTION 1 Which of the following compounds has an activating group? A. Benzoic acid B. None in the answer C. Bromobenzene D. Diphenylamine QUESTION 2 Which of the following compounds has a deactivating group attached to the benzene ring? A. Toluene B. Acetanilide C. Phenol D. Salicyclic Acidarrow_forwardWhat are the best starting material and reagents needed to make N-methylpropanamide? a. propanol and H2CrO7 b. propanoic acid, methylamine c. propanoic acid, methylamine, and high heat d. propene, H2SO4, H2O, and high heat e. methanoic acid, propylamine, and high heatarrow_forward
- Reduction of an alkyl azide results in the formation of —-. A. an imine B. an oxime C. a tertiary amine D. a secondary amine E. a primary aminearrow_forward10. Under certain conditions interacts with sodium nitrite: A. Resorcinol B. Nicotinic acid C. Butadion D. Morphine hydrochloride E. Benzoic acidarrow_forwardDescribe the reaction when a. Formaldehyde mixed with a fainty pink solution of potassium permanganate with a few drops of sulfuric acid. b. Acetaldehyde mixed with a fainty pink solution of potassium permanganate with a few drops of sulfuric acid. c. Benzaldehyde mixed with a fainty pink solution of potassium permanganate with a few drops of sulfuric acid.arrow_forward
- 45. Which of the following compounds has the lowest water solubility? a. butanal b. heptanal c. hexanal d. pentanal 48. Which of the following compounds is the most soluble in water? a. butanal b. heptanal c. hexanal d. pentanal 56. Which of the following compounds will undergo oxidation using potassium dichromate to form a carboxylic acid? A a. A and B only b. A and C only c. B only d. C only H B o Carrow_forward2. Draw out the following compounds. a. N-methylaniline b. Triisopropylamine c. N,N-dipropylhexylamine d. 1,5-pentanediamine (Also known as Cadaverine for its smell)arrow_forwardWhat are the functional groups present in this antibacterial antibiotic? A. Nitro, phenyl, amine, carbonyl, hydroxyl B. Nitro, phenyl, amine, carbonyl, C. Nitro, phenyl, amine, hydroxyl D. Nitro, phenol, amine, carbonyl, hydroxyl A brief explanation would be highly appreciated + upvotearrow_forward
- 1. A pleasant smelling liquid having a boiling point of 101°C. What is the structure of this compound? Major IR Absorptions Major lons in the MS 2880-2980 cm-¹ 29 41 1737 (str) 1194 (str) 1166 (str) 56 57 (base) 73 85 101 (small) 116 (molecular ion) 100 1737 500 vavenumber cm-1 157 Transmittance %T ģ 0. liquid film sample 100 80 60 4000 Relative Intensity 9 20 0 10 20 2977 9000 2876 30 4. 40 2000 50 56 60 m/z 70 1500 73 80 1194 85 1166 90 1000 101 repla 100 110 M: 116arrow_forwardErythronolide B is the biological precursor of erythromycin, a broad-spectrum antibiotic. What functional group Erythronolide B does contain? a. b. H₂CH₂C C. H₂C 1 H₂C 2 3 4 O Amide d. Amine OH Erythronolide B Ketone Aldehyde a CH₂ b C d CH₂ OH JCH₂ 'OH OHarrow_forwardDraw a structure corresponding to each name. a. 2,4-dimethyl-3-hexanamine b. N-methylpentylamine c. N-isopropyl-p-nitroaniline d. N-methylpiperidine e. N,N-dimethylethylamine f. 2-aminocyclohexanone g. N-methylaniline h. m-ethylanilinearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



