
Chemical Principles
8th Edition
ISBN: 9781305581982
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 147AE
The figure below represents part of the emission spectrumfor a one-electron ion in the gas phase. All the linesresult from electronic transitions from excited states tothe
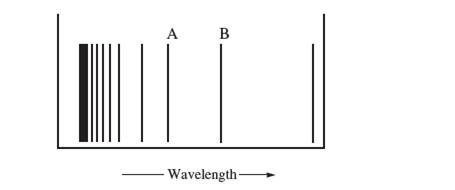
a. What electronic transitions correspond to lines Aand B?
b. If the wavelength of line B is 142.5 nm, calculate the wavelength of line A.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Chemical Principles
Ch. 12 - Prob. 1DQCh. 12 - Prob. 2DQCh. 12 - Prob. 3DQCh. 12 - The first four ionization energies for elements X...Ch. 12 - Prob. 5DQCh. 12 - Prob. 6DQCh. 12 - Prob. 7DQCh. 12 - Prob. 8DQCh. 12 - Prob. 9DQCh. 12 - Prob. 10DQ
Ch. 12 - Prob. 11DQCh. 12 - Prob. 12DQCh. 12 - Prob. 13DQCh. 12 - Prob. 14DQCh. 12 - Prob. 15DQCh. 12 - Prob. 16DQCh. 12 - Prob. 17DQCh. 12 - Is the following statement true or false: The...Ch. 12 - Which is higher in energy: the 2s or 2p orbital in...Ch. 12 - Prove mathematically that it is more energetically...Ch. 12 - Microwave radiation has a wavelength on the order...Ch. 12 - Consider the following waves representing...Ch. 12 - Prob. 23ECh. 12 - Human color vision is “produced” by the nervous...Ch. 12 - One type of electromagnetic radiation has a...Ch. 12 - Carbon absorbs energy at a wavelength of 150. nm....Ch. 12 - Prob. 27ECh. 12 - X rays have wavelengths on the order of 110-10m...Ch. 12 - The work function of an element is the energy...Ch. 12 - Ionization energy is the energy required to remove...Ch. 12 - It takes 208.4 kJ of energy to remove 1 mole of...Ch. 12 - What experimental evidence supports the quantum...Ch. 12 - Explain the photoelectric effect.Ch. 12 - Calculate the de Broglie wavelength for each of...Ch. 12 - Neutron diffraction is used in determining the...Ch. 12 - Calculate the velocities of electrons with de...Ch. 12 - An atom of a particular element is traveling at 1%...Ch. 12 - Characterize the Bohr model of the atom. In the...Ch. 12 - Prob. 39ECh. 12 - Consider only the transitions involving the first...Ch. 12 - Calculate the longest and shortest wavelengths of...Ch. 12 - Prob. 42ECh. 12 - Assume that a hydrogen atom’s electron has been...Ch. 12 - What is the maximum wavelength of light capable...Ch. 12 - An electron is excited from the ground state to...Ch. 12 - Does a photon of visible light (=400700nm)...Ch. 12 - An excited hydrogen atom emits light with a...Ch. 12 - An excited hydrogen atom with an electron in the n...Ch. 12 - Consider an electron for a hydrogen atom in an...Ch. 12 - Prob. 50ECh. 12 - One of the emission spectral lines for Be3+ has a...Ch. 12 - The Heisenberg uncertainty principle can be...Ch. 12 - Using the Heisenberg uncertainty principle,...Ch. 12 - We can represent both probability and radial...Ch. 12 - Prob. 55ECh. 12 - Calculate the wavelength of the electromagnetic...Ch. 12 - An electron in a one-dimensional box requires a...Ch. 12 - An electron in a 10.0-nm one-dimensional box is...Ch. 12 - Prob. 59ECh. 12 - What is the total probability of finding a...Ch. 12 - Which has the lowest (ground-state) energy, an...Ch. 12 - What are quantum numbers? What information do...Ch. 12 - How do 2p orbitals differ from each other? How do...Ch. 12 - Identify each of the following orbitals, and...Ch. 12 - Which of the following orbital designations are...Ch. 12 - Prob. 66ECh. 12 - The following sets of quantum numbers are not...Ch. 12 - How many orbitals can have the designation 5p,...Ch. 12 - How many electrons in an atom can have the...Ch. 12 - Prob. 70ECh. 12 - Prob. 71ECh. 12 - From the diagrams of 2p and 3p orbitals in Fig....Ch. 12 - Prob. 73ECh. 12 - Prob. 74ECh. 12 - Total radial probability distributions for the...Ch. 12 - The relative orbital levels for the hydrogen atom...Ch. 12 - What is the difference between core electrons and...Ch. 12 - Prob. 78ECh. 12 - Prob. 79ECh. 12 - The elements of Si, Ga, As, Ge, Al, Cd, S, and Se...Ch. 12 - Write the expected electron configurations for the...Ch. 12 - Write the expected electron configurations for...Ch. 12 - Prob. 83ECh. 12 - Using Fig. 12.29, list elements (ignore the...Ch. 12 - Prob. 85ECh. 12 - Prob. 86ECh. 12 - Prob. 87ECh. 12 - Prob. 88ECh. 12 - Prob. 89ECh. 12 - Prob. 90ECh. 12 - Prob. 91ECh. 12 - Prob. 92ECh. 12 - Prob. 93ECh. 12 - Prob. 94ECh. 12 - Prob. 95ECh. 12 - A certain oxygen atom has the electron...Ch. 12 - Prob. 97ECh. 12 - Prob. 98ECh. 12 - Prob. 99ECh. 12 - Explain why the first ionization energy tends to...Ch. 12 - Prob. 101ECh. 12 - The radius trend and the ionization energy trend...Ch. 12 - Prob. 103ECh. 12 - Prob. 104ECh. 12 - In each of the following sets, which atom or ion...Ch. 12 - Prob. 106ECh. 12 - Prob. 107ECh. 12 - Prob. 108ECh. 12 - Prob. 109ECh. 12 - Prob. 110ECh. 12 - Prob. 111ECh. 12 - Consider the following ionization energies for...Ch. 12 - Prob. 113ECh. 12 - Prob. 114ECh. 12 - Prob. 115ECh. 12 - Prob. 116ECh. 12 - Prob. 117ECh. 12 - Prob. 118ECh. 12 - Prob. 119ECh. 12 - Prob. 120ECh. 12 - Prob. 121ECh. 12 - Prob. 122ECh. 12 - Prob. 123ECh. 12 - Prob. 124ECh. 12 - Prob. 125ECh. 12 - Prob. 126ECh. 12 - Prob. 127ECh. 12 - Prob. 128AECh. 12 - Prob. 129AECh. 12 - Prob. 130AECh. 12 - Prob. 131AECh. 12 - Prob. 132AECh. 12 - Prob. 133AECh. 12 - Prob. 134AECh. 12 - Prob. 135AECh. 12 - Prob. 136AECh. 12 - Prob. 137AECh. 12 - Prob. 138AECh. 12 - Prob. 139AECh. 12 - An unknown element is a nonmetal and has a...Ch. 12 - Prob. 141AECh. 12 - Using data from this chapter, calculate the change...Ch. 12 - Answer the following questions, assuming that ms...Ch. 12 - Prob. 144AECh. 12 - Prob. 145AECh. 12 - Prob. 146AECh. 12 - The figure below represents part of the emission...Ch. 12 - Prob. 148AECh. 12 - Prob. 149AECh. 12 - Prob. 150AECh. 12 - Prob. 151AECh. 12 - Prob. 152AECh. 12 - Prob. 153AECh. 12 - Identify the following three elements. a. The...Ch. 12 - Prob. 155AECh. 12 - Prob. 156AECh. 12 - Prob. 157AECh. 12 - Prob. 158CPCh. 12 - The ground state ionization energy for the one...Ch. 12 - When the excited electron in a hydrogen atom falls...Ch. 12 - Prob. 161CPCh. 12 - The following numbers are the ratios of second...Ch. 12 - Prob. 163CPCh. 12 - Prob. 164CPCh. 12 - Prob. 165CPCh. 12 - Prob. 166CPCh. 12 - The ionization energy for a 1s electron in a...Ch. 12 - Without looking at data in the text, sketch a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What type of relationship (direct or inverse) e xists between wavelength, frequency, and photon energy? What does a photon energy unit of a joule equal?arrow_forwardThe figure below represents part of the emission spectrum for a one-electron ion in the gas phase. All the lines result from electronic transitions from excited states to the n 3 state. (See Exercise 174.) a. What electronic transitions correspond to lines A and B? b. If the wavelength of line B is 142.5 nm, calculate the wavelength of line A.arrow_forward6.29 A mercury atom emits light at many wavelengths, two of which are at 435.8 and 546.1 nm. Both of these transitions are to the same final state. (a) What is the energy difference between the two states for each transition? (b) lf a transition between the two higher energy states could be observed, what would be the frequency of the light?arrow_forward
- Calculate the wavelength of the Balmer line of the hydrogen spectrum in which the initial n quantum number is 5 and the final n quantum number is 2.arrow_forwardPotassium atoms in a flame emit light as they undergo transitions from one energy level to another that is 4.91019J lower in energy. Calculate the wavelength of light emitted and, by referring to Figure 4.3, predict the color visible in the flame.arrow_forwardAs the weapons officer aboard the Srarship Chemistry, it is your duty to configure a photon torpedo to remove an electron from the outer hull of an enemy vessel. You know that the work function (the binding energy of the electron) of the hull of the enemy ship is 7.52 1019 J. a. What wavelength does your photon torpedo need to be to eject an electron? b. You find an extra photon torpedo with a wavelength of 259 nm and fire it at the enemy vessel. Does this photon torpedo do any damage to the ship (does it eject an electron)? c. If the hull of the enemy vessel is made of the element with an electron configura tion of [Ar]4s13d10, what metal is this?arrow_forward
- What are the allowed values for each of the four quantum numbers: n, l, ml, and ms?arrow_forward6.9 If a string of decorative lights includes bulbs with wave-lengths of 480, 580, and 700 mm, what are the frequencies of the lights? Use Figure 6.6 to determine which colors are in the set.arrow_forwardA bright violet line occurs at 435.8 nm in the emission spectrum of mercury vapor. What amount of energy, in joules, must be released by an electron in a mercury atom to produce a photon of this light?arrow_forward
- A hydrogen atom in the ground stale absorbs a photon whose wavelength is 95.0 nm. The resulting excited atom then emits a photon of 1282 nm. What are the regions of the electromagnetic spectrum for the radiations involved in these transitions? What is the principal quantum number of the final state resulting from the emission from the excited atom?arrow_forward6.93 A mercury atom is initially in its lowest possible (or ground state) energy level. The atom absorbs a photon with a wavelength of 185 nm and then emits a photon with a frequency of 4.9241014HZ . At the end of this series of transitions, the atom will still be in an energy level above the ground state. Draw an energy-level diagram for this process and find the energy of this resulting excited state, assuming that we assign a value of E = 0 to the ground state. (This choice of E = 0 is not the usual convention, but it will simplify the calculations you need to do here.)arrow_forwardA baseball weighs 142 g. A professional pitcher throws a fast ball at a speed of 100 mph and a curve ball at 80 mph. What wavelengths are associated with the motions of the baseball? If the uncertainty in the position of the ball is 12 wavelength, which ball (fast ball or curve) has a more precisely known position? Can the uncertainty in the position of a curve ball be used to explain why batters frequently miss it?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
Quantum Mechanics - Part 1: Crash Course Physics #43; Author: CrashCourse;https://www.youtube.com/watch?v=7kb1VT0J3DE;License: Standard YouTube License, CC-BY