
(a)
Interpretation:
The detailed mechanism with the major product for the given reaction is to be drawn.
Concept introduction:
The acid-catalyzed hydration of an
Answer to Problem 12.42P
The detailed mechanism for the given reaction is
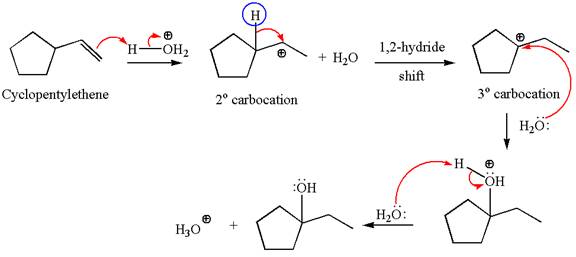
Explanation of Solution
The given reaction is
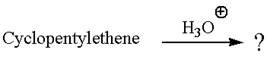
Cyclopentylethene, a terminal alkene, in presence of H3O+ undergoes an acid catalyzed hydration where the water molecule adds across the C=C bond and produces the corresponding alcohol product though formation of a carbocation intermediate.
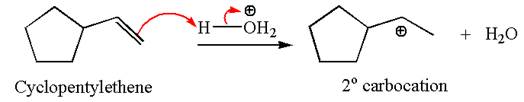
The first step is the formation of a secondary carbocation by proton transfer reaction. The proton transfers to the less substituted double bonded carbon.
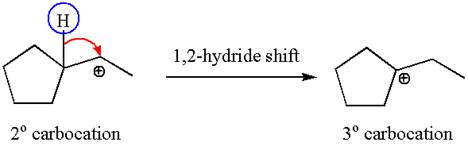
The secondary carbocation can be rearranged to a more stable tertiary carbocation by 1, 2- hydride shift.
In the second step, the water molecule acts as a nucleophile and attacks the tertiary carbocation, forming a tertiary alcohol product, followed by deprotonation of the positively charged oxygen.
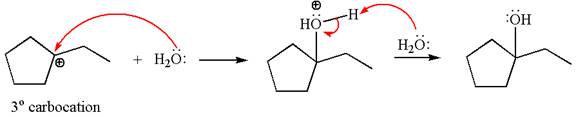
The detailed mechanism for the given reaction is drawn by suggesting that the reaction occurs through carbocation rearrangement.
(b)
Interpretation:
The detailed mechanism with major product for the given reaction is to be drawn.
Concept introduction:
The oxymercuration-reduction is also the reaction of addition of water across the C=C bond. The alkene is first reacted with mercury (II) acetate, Hg(OAc)2, in water–tetrahydrofuran (THF) solution, and that is followed by reduction with sodium borohydride, NaBH4. The mechanism follows the Markovnikov rule and adds OH to more substituted double bonded carbon. The reaction proceeds through the formation of a Mercurinium ion intermediate, and there is no formation of a carbocation. Thus, the rearrangement is not possible in oxymercuration-reduction.
Answer to Problem 12.42P
The detailed mechanism for the given reaction is
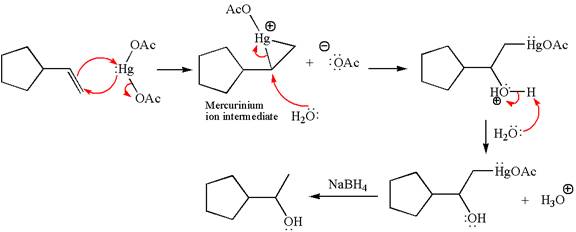
Explanation of Solution
The given reaction is
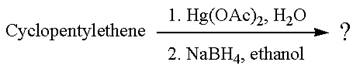
Cyclopentylethene, a terminal alkene, on reaction with mercury (II) acetate, Hg(OAc)2, in water, followed by NaBH4 in ethanol, undergoes oxymercuration-reduction.
In the first step, the electron rich C=C bond attacks the electrophilic Hg whose electron pair forms a bond with the carbon producing three membered ring, called mercurinium ion intermediate.
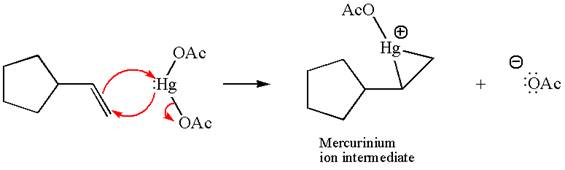
In the second step, the water molecule acts as a nucleophile and, according to Markovnikov rule, attacks the most substituted side to open the three-membered ring, followed by deprotonation of the positively charged oxygen atom.
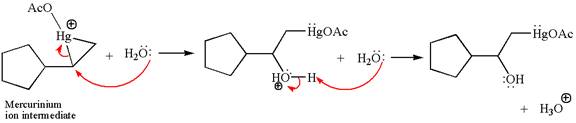
The product formed in the previous step is then subjected to reduction with sodium borohydride, NaBH4 where the substituent HgOAc is replaced by H.
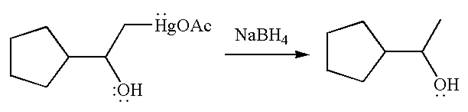
The detailed mechanism for the given reaction is drawn by suggesting that the reaction occurred through formation of mercurinium ion intermediate without rearrangement.
(c)
Interpretation:
The detailed mechanism with major product for the given reaction is to be drawn.
Concept introduction:
The acid-catalyzed hydration of an alkene is the electrophilic addition of water across the C=C bond in an acidic condition. The electrophilic addition reaction of an alkene occurs through the formation of a more stable carbocation. The reaction proceeds with proton transfer reaction to form a stable carbocation followed by the action of water as a nucleophile, forming the corresponding alcohol. The order of stability of carbocation is CH3 < 1o < 2o < 3o. The carbocation can be rearranged by 1, 2- hydride or 1, 2- methyl shift to form a more stable carbocation.
Answer to Problem 12.42P
The detailed mechanism for the given reaction is
Explanation of Solution
The given reaction is
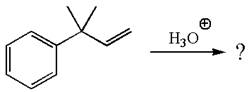
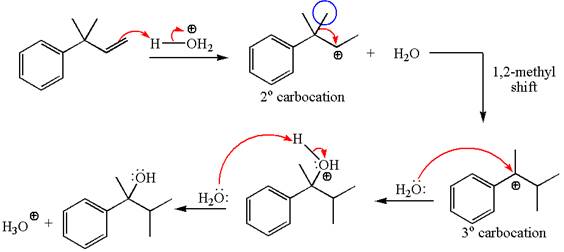
The given substrate, a terminal alkene, in the presence of H3O+, undergoes an acid catalyzed hydration where the water molecule adds across the C=C bond and producea the corresponding alcohol product though formation of a carbocation intermediate.
The first step is the formation of a secondary carbocation by proton transfer reaction. The proton transfers to the less substituted double bonded carbon.
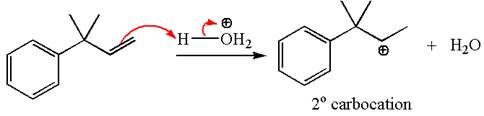
The secondary carbocation can be rearranged to a more stable tertiary as well as resonance stabilized carbocation by 1, 2- methyl shift.
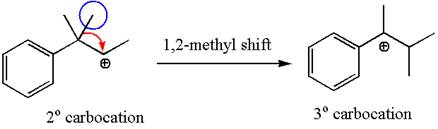
In the second step, the water molecule acts as a nucleophile and attacks the tertiary carbocation, forming a tertiary alcohol product, followed by deprotonation of the positively charged oxygen.
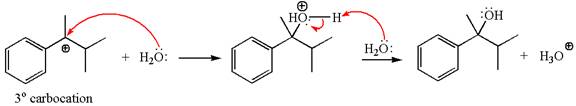
The detailed mechanism for the given reaction is drawn by suggesting that the reaction occurred through carbocation rearrangement.
(d)
Interpretation:
The detailed mechanism with major product for the given reaction is to be drawn.
Concept introduction:
The oxymercuration-reduction is also the reaction of addition of water across the C=C bond. The alkene is first reacted with mercury (II) acetate, Hg(OAc)2, in water–tetrahydrofuran (THF) solution, and that is followed by reduction with sodium borohydride, NaBH4. The mechanism follows the Markovnikov rule and adds OH to the more substituted double bonded carbon. The reaction proceeds through formation of a Mercurinium ion intermediate, and there is no formation of a carbocation. Thus, rearrangement is not possible in oxymercuration-reduction.
Answer to Problem 12.42P
The detailed mechanism for the given reaction is
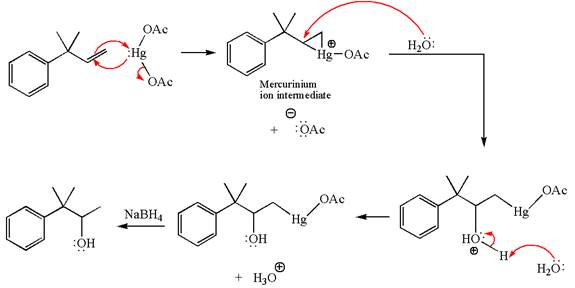
Explanation of Solution
The given reaction is
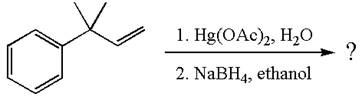
The given substrate, a terminal alkene, on reaction with mercury (II) acetate, Hg(OAc)2, in water followed by NaBH4 in ethanol, undergoes oxymercuration-reduction.
In the first step, the electron rich C=C bond of alkene attacks the electrophilic Hg whose electron pair forms a bond with the carbon producing three-membered ring, called mercurinium ion intermediate.
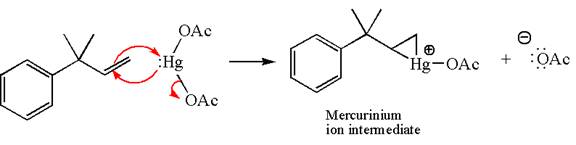
In the second step, the water molecule acts as a nucleophile and, according to Markovnikov rule, attacks the most substituted side to open the three-membered ring, followed by deprotonation of the positively charged oxygen atom.
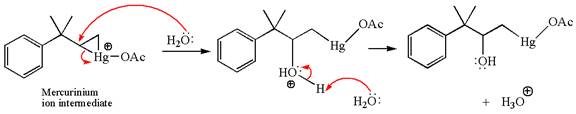
The product formed in the previous step is then subjected to reduction with sodium borohydride, NaBH4, where the substituent HgOAc is replaced by H.
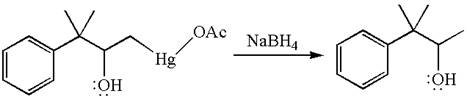
The detailed mechanism for the given reaction is drawn by suggesting that the reaction occurred through formation of mercurinium ion intermediate without rearrangement.
Want to see more full solutions like this?
Chapter 12 Solutions
ORGANIC CHEM PRINC & MECH (BUNDLE)
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
