
(a)
Interpretation:
The structural formula for the
Concept introduction:
The addition reactions are defined as the formation of one product by the combination of two or more products. For example, in halogenation, the addition of a halogen molecule results in the reduction of carbon-carbon double bond to carbon-carbon single bond and formation of
Answer to Problem 12.27E
The structural formula for the alkene with molecular formula
Explanation of Solution
The reaction is shown below.
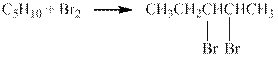
Figure 1
The product
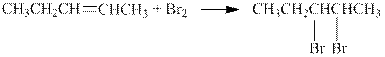
Figure 2
The structural formula for the alkene with molecular formula
(b)
Interpretation:
The structural formula for the alkene with molecular formula
Concept introduction:
The addition reactions are defined as the formation of one product by the combination of two or more products. For example, in halogenation, the addition of a halogen molecule results in the reduction of carbon-carbon double bond to carbon-carbon single bond and formation of haloalkane.
Answer to Problem 12.27E
The structural formula for the alkene with molecular formula
Explanation of Solution
The reaction is shown below.
The product in the given reaction is pentane which can be prepared by the hydrogenation on the
The structural formula for the alkene with molecular formula
(c)
Interpretation:
The structural formula for the alkene with molecular formula
Concept introduction:
The Markovnikov’s rule states that the addition of the
Answer to Problem 12.27E
The structural formula for the alkene with molecular formula
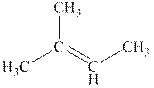
Explanation of Solution
The reaction is shown below.
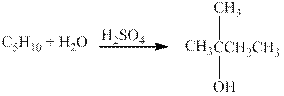
Figure 2
The product in the reaction is
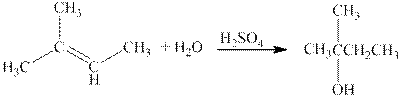
Figure 3
The structural formula for the alkene with molecular formula
(d)
Interpretation:
The structural formula for the alkene with molecular formula
Concept introduction:
The Markovnikov’s rule states that the addition of the
Answer to Problem 12.27E
The structural formula for the alkene with molecular formula
Explanation of Solution
The given reaction is shown below.

Figure 4
The product in the given reaction is
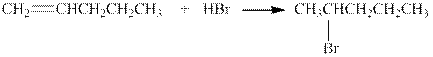
Figure 5
The structural formula for the alkene with molecular formula
Want to see more full solutions like this?
Chapter 12 Solutions
Chemistry For Today: General, Organic, And Biochemistry, Loose-leaf Version
- Explain why unbranched alkenes can form geometric isomers while unbranched alkanes cannot. Does this explanation involve the macroscopic domain or the microscopic domain?arrow_forwardYields in organic reactions are sometimes low. What is the percent yield of a process that produces 13.0 g of ethyl acetate from 10.0 g of CH3CO2H?arrow_forwardWhat is a hydrocarbon? What is the difference between a saturated hydrocarbon and an unsaturated hydrocarbon? Distinguish between normal and branched hydrocarbons. What is an alkane? What is a cyclic alkane? What are the two general formulas for alkanes? What is the hybridization of carbon atoms in alkanes? What are the bond angles in alkanes? Why are cyclopropane and cyclobutane so reactive? The normal (unbranched) hydrocarbons are often referred to as straight-chain hydrocarbons. What does this name refer to? Does it mean that the carbon atoms in a straight-chain hydrocarbon really have a linear arrangement? Explain. In the shorthand notation for cyclic alkanes, the hydrogens are usually omitted. How do you determine the number of hydrogens bonded to each carbon in a ring structure?arrow_forward
- Consider a sample of a hydrocarbon at 0.959 atm and 298 K. Upon combusting the entire sample in oxygen, you collect a mixture of gaseous carbon dioxide and water vapor at 1.51 atm and 375 K. This mixture has a density of 1.391 g/L and occupies a volume four times as large as that of the pure hydrocarbon. Determine the molecular formula of the hydrocarbon and name it.arrow_forward29) When C3H8 hydrocarbon combustion reaction occurs the products are: a. 4CO2 + 3H2O b. ЗСО2 + 4H20 с. 2СО2 + ЗН20 d. 3CO + 4H2Oarrow_forward12. Write structural formulas for the following alkene names. a. hept-2-ene b. hex-2-ene 6,7-dimethyloct-1-ene d. 2,4-dimethyloct-4-ene с. 3-ethylnon-2-ene е.arrow_forward
- 1. What is the alkane product from the reaction of C7H13COONa and NaOH? a. pentane b. hexane c. butane d. heptane 2. What is the resulting alkane if we have C5H11F and a C5H11F as reactants in Wurtz synthesis? a. hexane b. octane c. nonane d. decane 3.What is the resulting alkane if we have 2C2H5Cl as reactants in Wurtz Synthesis reaction? a. ethane b. butane c. hexane d. octanearrow_forward12. Which of the following structural formulas shows a compound that is an isomer of butane? C. e. H 110-4 H H III H - H c. alkyl halides d. amines e. ethers H н-с HIC CIC-H 'H HIC-H T 4 * ΗΗΗΗ H-C-C-C-C-O-H ΗΗΗΗ b. H-C # H- C-H H H d. H HIĊ-C= H H H 13. Which class of organic molecules can form directly from alkanes? a. alcohols b. ketones H -C-Harrow_forward12. Which of the following structural formulas shows a compound that is an isomer of butane? C. e. H 110-4 H H III H - H c. alkyl halides d. amines e. ethers H н-с HIC CIC-H 'H HIC-H T 4 * ΗΗΗΗ H-C-C-C-C-O-H ΗΗΗΗ b. H-C # H- C-H H H d. H HIĊIC= H H H 13. Which class of organic molecules can form directly from alkanes? a. alcohols b. ketones H -C-Harrow_forward
- A. Complete and balance the following combustion reactions. Assume that each hydrocarbon is converted completely to carbon dioxide and water. 1. Propane + O2 → 2. Cyclohexane + 02→ 3.2-Methylpentane + O2 → B. Following are structural formulas and heats of combustion of acetaldehyde and ethylene oxide. Which of these compounds is the more stable? Explain. CH-CH H,C-CH2 Acetaldehyde -1164 kJ (-278.8 kcal)/mol Ethylene oxide -1264 kJ (-302.1 kcal)/molarrow_forwardShow how to convert 1- Butene into these compounds. a. Butane b. 2- Butanol c. 2- Bromobutane d. 1,2- Dibromobutanearrow_forwardThe oxidation of an alkene with KMNO4 produces O A. an epoxide O B. an aldehyde O C. an acid O D. a diolarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





