
(a)
To determine: The characteristic infrared absorptions of the
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule are to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or
(a)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
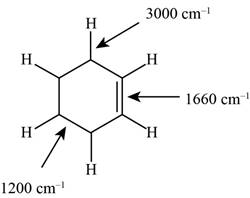
Figure 1
Explanation of Solution
The structure of the given molecule is,
Figure 2
The possibilities of the IR stretching frequencies for the given molecule are shown as,
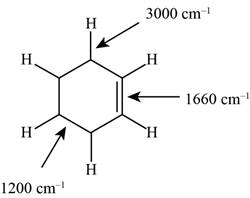
Figure 1
So, the stretching frequencies of
(b)
To determine: The characteristic infrared absorptions of the functional groups in the given molecule.
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule are to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or wavelength of light. Alkanes, alkenes and alkynes have characteristic
(b)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
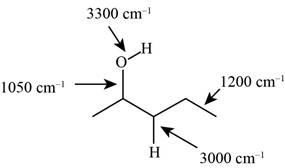
Figure 3
Explanation of Solution
The structure of the given molecule is,
Figure 4
The possibilities of the IR stretching frequencies for the given molecule are shown as,
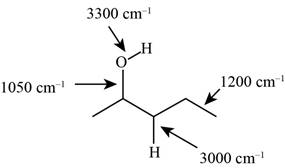
Figure 3
So, the stretching frequencies of
(c)
To determine: The characteristic infrared absorptions of the functional groups in the given molecule.
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule are to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or wavelength of light. Alkanes, alkenes and alkynes have characteristic
(c)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
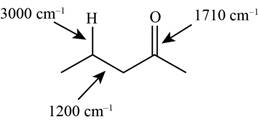
Figure 5
Explanation of Solution
The structure of the given molecule is,
Figure 6
The possibilities of the IR stretching frequencies for the given molecule are shown as,
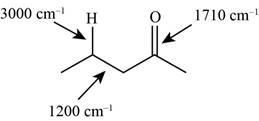
Figure 5
So, the stretching frequencies of
(d)
To determine: The characteristic infrared absorptions of the functional groups in the given molecule.
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule are to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or wavelength of light. Alkanes, alkenes and alkynes have characteristic
(d)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
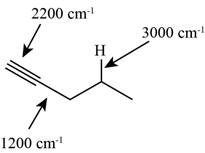
Figure 7
Explanation of Solution
The structure of the given molecule is,
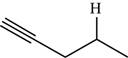
Figure 8
The possibilities of the IR stretching frequencies for the given molecule are shown as,

Figure 7
So, the stretching frequencies of
(e)
To determine: The characteristic infrared absorptions of the functional groups in the given molecule.
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule are to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or wavelength of light. Alkanes, alkenes and alkynes have characteristic
(e)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
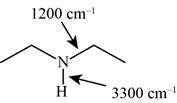
Figure 9
Explanation of Solution
The structure of the given molecule is,
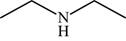
Figure 10
The possibilities of the IR stretching frequencies for the given molecule are shown as,
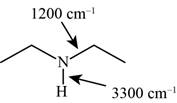
Figure 9
So, the stretching frequencies of
(f)
To determine: The characteristic infrared absorptions of the functional groups in the given molecule.
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule is to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or wavelength of light. Alkanes, alkenes and alkynes have characteristic
(f)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
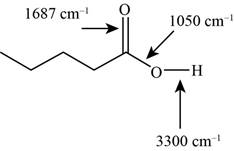
Figure 11
Explanation of Solution
The structure of the given molecule is,
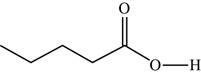
Figure 12
The possibilities of the IR stretching frequencies for the given molecule are shown as,

Figure 11
So, the stretching frequencies of
(g)
To determine: The characteristic infrared absorptions of the functional groups in the given molecule.
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule are to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or wavelength of light. Alkanes, alkenes and alkynes have characteristic
(g)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
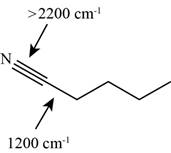
Figure 13
Explanation of Solution
The structure of the given molecule is,
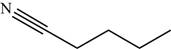
Figure 14
The possibilities of the IR stretching frequencies for the given molecule are shown as,
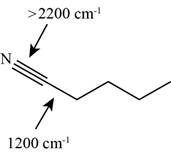
Figure 13
So, the stretching frequencies of
(h)
To determine: The characteristic infrared absorptions of the functional groups in the given molecule.
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule are to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or wavelength of light. Alkanes, alkenes and alkynes have characteristic
(h)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
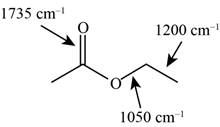
Figure 15
Explanation of Solution
The structure of the given molecule is,
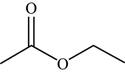
Figure 16
The possibilities of the IR stretching frequencies for the given molecule are shown as,
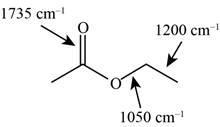
Figure 15
So, the stretching frequencies of
(i)
To determine: The characteristic infrared absorptions of the functional groups in the given molecule.
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule are to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or wavelength of light. Alkanes, alkenes and alkynes have characteristic
(i)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
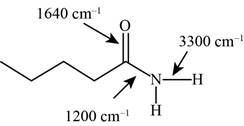
Figure 17
Explanation of Solution
The structure of the given molecule is,
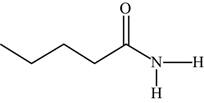
Figure 18
The possibilities of the IR stretching frequencies for the given molecule are shown as,

Figure 17
So, the stretching frequencies of
Want to see more full solutions like this?
Chapter 12 Solutions
ORGANIC CHEMISTRY
- The structure of the molecule cyclohexene is Does the absorption of ultraviolet light by cyclohexene occur at longer or at shorter wavelengths than the absorption by benzene? Explain.arrow_forward12.arrow_forwardThe functional groups in an organic compound can frequently be deduced from its infrared absorption spectrum. A compound containing C, H, and O exhibits broad absorption at 3450 cm-1 (m) and an intense band at 1725, plus a band at 1100 cm-1 (m).Relative absorption intensity: (s)=strong, (m)=medium, (w)=weak. What functional class(es) does the compound belong to? List only classes for which evidence is given here. Attach no significance to evidence not cited explicitly.Do not over-interpret exact absorption band positions. None of your inferences should depend on small differences like 10 to 20 cm-1. The functional class(es) of this compound is(are)fill in the blank 1.(Enter letters from the table below, in any order, with no spaces or commas.) a. alkane (List only if no other functional class applies.) b. alkene h. amine c. terminal alkyne i. aldehyde or ketone d. internal alkyne j. carboxylic acid e. arene k. ester f. alcohol l. nitrile g. etherarrow_forward
- When 2-methylpropane is monochlorinated in the presence of light at room temperature, 36% of the product is 2-chloro-2-methylpropane and 64% is 1-chloro-2-methylpropane. From these data, calculate how much easier it is to remove a hydrogen atom from a tertiary carbon than from a primary carbon under these conditions.arrow_forwardIndicate the functional groups found in the IR signals of: 1. Hexene 2. Cyclohexene 3. Toluene 4. Benzenearrow_forwardAnalyze the IR spectra to determine the identity of the unknown solid organic compound. Identify the peaks in the spectra and show each functional group present in the organic compound. The list of all the possible organic compounds are as follows: Cumene, 3-Methy-2-butanone, Phenethyl alcohol, Isopropyl Acetate, Ethel benzoate, 3-phenylpropionaldehyde, isobutyl cinnamate, cyclamen aldehyde, Styrallyl acetate, Butyl butyrate, propiophenon, phenylacetylene, Cis-Hexenyl-3-Acetate, Benzyl propionate, 3-(p-ethyl-phenyl)-2,2-dimethyl propionaldehyde, 2-methoxy-4-ethyl-phenol, P-ethylphenol, diphenyl amine, 4-phenyl-3-buten-2-one, Diethyl Terphthalate, glutaric acid, cinnamic acid, P- ethylbenzoic acid, Coumarin, vanillin, (2,2,2-trichloro-1-phenylethyl)acetate, 4-(p-hydroxyphenyl)-2-butanone, 2-ethoxy-5-prop-1-enylphenol, t-butyl-phenol, Thymol, Calone, Syringol, Syringaldehyde. Borderline MP unknowns: Styrallyl alcohol, 1,1-dimethyl-2-phenethyl acetate, 1,1-dimethyl-2-phenyl ethanol.arrow_forward
- The IR spectrum of a sample contains absorptions at 3050, 2950, and 1620 cm-1. Towhat class of organic compound does this sample most likely belong? ExplainA) alkaneB) alkeneC) alkyneD) esterE) alcoholarrow_forwardRemaining Time: 1 hour, 11 minutes, 56 seconds. * Question Completion Status: A Moving to the next question prevents changes to this answer. Question 8 How many unhybridized p-orbitals are in the carbon label b in the structure below? O A. 1 B. 2 DC. 3 OD.4 O E. O 4. Unit 4 - Diastereo..m4v Deen fle 3. Unit 4 - R And S..m4v Open file 1. Unit 4 - Introduct..m4v Cpen file earch a 99arrow_forward1. True or False a. UV-Vis spectroscopy is necessary for the analysis of organic compounds because most organic compounds are colored green in the presence of an extracting solvent. (T/F) b. The spectrophotometer is able to detect and report Absorbance values of a compound based on the compound's ability to absorb radiation or emit radiation. (T/F) c. A common electronic transition for an organic compound is the electron shifting from a bonding orbital to a nonbonding orbital. (T/F)arrow_forward
- The functional groups in an organic compound can frequently be deduced from its infrared absorption spectrum. A compound exhibits strong, broad absorption between 3300 and 3500 and at 1050 cm-1. Relative absorption intensity: (s)=strong, (m)=medium, (w)=weak. What functional class(es) does the compound belong to? List only classes for which evidence is given here. Attach no significance to evidence not cited explicitly. Do not over-interpret exact absorption band positions. None of your inferences should depend on small differences like 10 to 20 cm-1. The functional class(es) of this compound is(are) -(Enter letters from the table below, in any order, with no spaces or commas.) a. alkane (List only if no other functional class applies.) b. alkene c. terminal alkyne d. internal alkyne h. amine i. aldehyde or ketone j. carboxylic acid k. ester I. nitrile e. arene f. alcohol g. etherarrow_forward4) Compare the IR spectra of both isomers and show the bond vibrations for functional groups, decide which one is nitro or nitrito isomer. bom 72.00- 64.00- 56.00- 48.00- 40.00- 32.00- 24.00- 16.00- 8.00- 4000 3600 3200 2800 2400 2000 1600 1200 800 72.00- 64.00- 56.00- 48.00- 40 00- 32.00 - 24.00- 16.00- 8.00- 4000 3600 3200 2800 2400 2000 1600 1200 800arrow_forwardHere is the chemical structure of 2-bromobutane: Н Η Н Н HHHH C C- C C-H H :Br Η Н .. Decide whether each molecule in the table below is another molecule of 2-bromobutane, a molecule of an isomer of 2-bromobutane, or a molecule of an entirely different compound. molecule CH₂ CH₂-CH₂-CH-Br CH₂ CH₂ -CH₂ Br HI H H H Η H_ H-C- C-C- H-C-H H H relationship to 2-bromobutane (Choose one) (Choose one) Br: a molecule of an isomer of 2-bromobutane ▼arrow_forward

 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


