
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 11.9, Problem 19P
Interpretation Introduction
Interpretation:
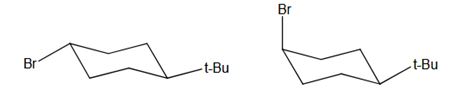
In which isomer the E2 elimination occurs faster is interpreted. The more stable chair confirmation is drawn to explain the reaction.
Concept introduction:
If the leaving group and hydrogen are trans diaxial only the E2 elimination occurs in cyclohexane rings. The E2 elimination occurs by Zaitsevs rule.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Which of the two isomers would you expect to undergo E2 elimination faster? trans-1-bromo- 4-tert-butylcyclohexane or cis-1-bromo-4-tert-butylcyclohexane? Draw each molecule in its more stable chair conformation. Provide an explanation to your answer.
Trans-1-bromo-2-methylcyclohexane will yield a non-Zaitsev elimination product (3-methylcyclohexene) upon reaction with KOH. Show this reaction by drawing the chair conformations of the reactant and product. Include the curved arrows and explain why the product is not a non-Zaitsev product.
2) Draw Newman projections of all staggered conformations of (S)-butan-2-ol. Clearly mark the
highest energy conformation.
3) Draw both chair conformations of (1S,3R)-1-chloro-3-isopropylcyclohexane. Clearly mark the
most stable conformation, and also the conformation which enables E2 elimination.
Chapter 11 Solutions
Organic Chemistry
Ch. 11.1 - Prob. 1PCh. 11.2 - Prob. 2PCh. 11.2 - Prob. 3PCh. 11.3 - Prob. 4PCh. 11.3 - Prob. 5PCh. 11.3 - Rank the following compounds in order of their...Ch. 11.3 - Organic solvents like benzene, ether, and...Ch. 11.4 - Prob. 8PCh. 11.4 - Prob. 9PCh. 11.4 - Prob. 10P
Ch. 11.5 - Rank the following substances in order of their...Ch. 11.5 - 3-Bromo-1-butene and 1-bromo-2-butene undergo SN1...Ch. 11.5 - Prob. 13PCh. 11.6 - Review the mechanism of geraniol biosynthesis...Ch. 11.7 - Prob. 15PCh. 11.7 - What alkyl halides might the following alkenes...Ch. 11.8 - Prob. 17PCh. 11.8 - Prob. 18PCh. 11.9 - Prob. 19PCh. 11.12 - Prob. 20PCh. 11.SE - Prob. 21VCCh. 11.SE - From what alkyl bromide was the following alkyl...Ch. 11.SE - Prob. 23VCCh. 11.SE - Prob. 24VCCh. 11.SE - Prob. 25MPCh. 11.SE - Prob. 26MPCh. 11.SE - Prob. 27MPCh. 11.SE - Prob. 28MPCh. 11.SE - Prob. 29MPCh. 11.SE - Prob. 30MPCh. 11.SE - Prob. 31MPCh. 11.SE - Prob. 32MPCh. 11.SE - Metabolism of S-adenosylhomocysteine (Section...Ch. 11.SE - Reaction of iodoethane with CN- yields a small...Ch. 11.SE - One step in the urea cycle for ridding the body of...Ch. 11.SE - Prob. 36MPCh. 11.SE - Prob. 37MPCh. 11.SE - Propose a mechanism for the following reaction, an...Ch. 11.SE - Prob. 39APCh. 11.SE - The following Walden cycle has been carried out....Ch. 11.SE - Prob. 41APCh. 11.SE - Which reactant in each of the following pairs is...Ch. 11.SE - Prob. 43APCh. 11.SE - Prob. 44APCh. 11.SE - Prob. 45APCh. 11.SE - Prob. 46APCh. 11.SE - Prob. 47APCh. 11.SE - Prob. 48APCh. 11.SE - Propose structures for compounds that fit the...Ch. 11.SE - What products would you expect from the reaction...Ch. 11.SE - Prob. 51APCh. 11.SE - Prob. 52APCh. 11.SE - Prob. 53APCh. 11.SE - Prob. 54APCh. 11.SE - Prob. 55APCh. 11.SE - Order each of the following sets of compounds with...Ch. 11.SE - Order each of the following sets of compounds with...Ch. 11.SE - Prob. 58APCh. 11.SE - Prob. 59APCh. 11.SE - Ethers can often be prepared by SN2 reaction of...Ch. 11.SE - Show the stereochemistry of the epoxide (see...Ch. 11.SE - Prob. 62APCh. 11.SE - In addition to not undergoing substitution...Ch. 11.SE - The tosylate of (2R, 3S)-3-phenyl-2-butanol...Ch. 11.SE - Prob. 65APCh. 11.SE - Prob. 66APCh. 11.SE - Prob. 67APCh. 11.SE - Prob. 68APCh. 11.SE - Prob. 69APCh. 11.SE - (S)-2-Butanol slowly racemizes on standing in...Ch. 11.SE - Reaction of HBr with (R)-3-methyl-3-hexanol leads...Ch. 11.SE - Treatment of 1-bromo-2-deuterio-2-phenylethane...Ch. 11.SE - Prob. 73APCh. 11.SE - Prob. 74APCh. 11.SE - In light of your answer to Problem 11-74, explain...Ch. 11.SE - Prob. 76APCh. 11.SE - Compound X is optically inactive and has the...Ch. 11.SE - When a primary alcohol is treated with...Ch. 11.SE - Prob. 79APCh. 11.SE - Amines are converted into alkenes by a two-step...Ch. 11.SE - The antipsychotic drug flupentixol is prepared by...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Trans-1-bromo-2-methylcyclohexane will yield a non-Zaitsev elimination product (3-methylcyclohexene) upon reaction with KOH. Show this reaction by drawing the chair conformations of the reactant and product. Include the curved arrows and explain why the product is not a non-Zaitsev product.arrow_forwardPredict the stereochemistry for the following E2 reaction. Draw a Newmann Projection of the reactive conformation and the structure for only major product of the reactionarrow_forwardPlease answer poarrow_forward
- Draw the starting alkene that would lead to this the major product (and its enantiomer) under these conditions. Drawing H2 Pd/C OD0 000 F5 F4arrow_forwardDraw the Newman projection (sighting down this bond) of the conformation that will be capable of an E2 elimination. R/S stereochemistry is graaded. CH3 H. H. CH3 Please selec Br Harrow_forward11. Trans-1-bromo-2-methylcyclohexane will yield a non-Zaitsev elimination product (3- methylcyclohexene) upon reaction with KOH. Show this reaction by drawing the chair conformations of the reactant and product. Include the curved arrows and explain why the product is not a non-Zaitsev product. (arrow_forward
- Ketones react with alcohols to yield products called acetals. Why does the all-cis isomer of 4-tert-butyl-1,3-cyclohexanediol react readily with acetone and an acid catalyst to form an acetal, but other stereoisomers do not react? In formulating your answer, draw the more stable chair conformations of all four stereoisomers and the product acetal for each one.arrow_forward(a) When cis-1-bromo-2-methylcyclohexane undergoes an E2 reaction, two products (cycloalkenes) are formed. What are these two cycloalkenes, and which would you expect to be the major product? Write conformational structures showing how each is formed. (b) When rans-1-bromo-2-methylcyclohexane reacts in an E2 reaction, only one cyclo- alkene is formed. What is this product? Write conformational structures showing why it is the only product.arrow_forwardDraw each of the above molecules in a 3D perspective. Show all six-membered rings as chairs and all acyclic torsions in staggered conformation. If multiple conformations are possible, choose the best one.arrow_forward
- Acyclic conjugated dienes may exist in two conformations, as shown below. Based on differences in steric strain, which of the following dienes has the greatest preference for the s-trans conformation? * s-cis s-trans H;C / H;C- CH3 CH3 CH3 H;C- CH3arrow_forwardH2/Ptarrow_forward2. Our knowledge of chair conformations allows us to use them to determine a great deal about a reaction's mechanism. In particular, using a t-butyl group to 'fix' a cyclohexane in a single conformation can help us learn about the spatial requirements and energetics of a reaction. Consider the following two reaction products: a. NO NID ALOH 1) NaCN 2) H+ 1) NaBH4 2) H3O+ OH Group -H -CN -OH A Value (kcal/mol) Defined as 0 0.2 kcal/mol 1.0 kcal/mol Draw the most stable chair conformation for each of the two products. b. Based on the A values in the table above, do the two products have the most stable conformation at the new stereocenter?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning