
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 11.5, Problem 13P
Interpretation Introduction
a)
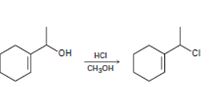
Interpretation:
The above substitution reaction belongs to SN1 type.
Concept introduction:
SN1 reaction occurs mostly in acidic condition. The OH group is first protonated by HCl. Spontaneous dissociation of the protonated alcohol occurs in a slow, rate determining step to yield a carbocation intermediate. The carbocation intermediate reacts with the chloride ion to yield the chloride product.
Interpretation Introduction
b)
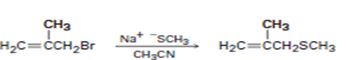
Interpretation:
The above substitution reaction belongs to SN2 type.
Concept introduction:
SN2 reaction occurs rapidly in
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Show mechanism..don't give Ai generated solution
Don't used Ai solution
Show work. Don't give Ai generated solution
Chapter 11 Solutions
Organic Chemistry
Ch. 11.1 - Prob. 1PCh. 11.2 - Prob. 2PCh. 11.2 - Prob. 3PCh. 11.3 - Prob. 4PCh. 11.3 - Prob. 5PCh. 11.3 - Rank the following compounds in order of their...Ch. 11.3 - Organic solvents like benzene, ether, and...Ch. 11.4 - Prob. 8PCh. 11.4 - Prob. 9PCh. 11.4 - Prob. 10P
Ch. 11.5 - Rank the following substances in order of their...Ch. 11.5 - 3-Bromo-1-butene and 1-bromo-2-butene undergo SN1...Ch. 11.5 - Prob. 13PCh. 11.6 - Review the mechanism of geraniol biosynthesis...Ch. 11.7 - Prob. 15PCh. 11.7 - What alkyl halides might the following alkenes...Ch. 11.8 - Prob. 17PCh. 11.8 - Prob. 18PCh. 11.9 - Prob. 19PCh. 11.12 - Prob. 20PCh. 11.SE - Prob. 21VCCh. 11.SE - From what alkyl bromide was the following alkyl...Ch. 11.SE - Prob. 23VCCh. 11.SE - Prob. 24VCCh. 11.SE - Prob. 25MPCh. 11.SE - Prob. 26MPCh. 11.SE - Prob. 27MPCh. 11.SE - Prob. 28MPCh. 11.SE - Prob. 29MPCh. 11.SE - Prob. 30MPCh. 11.SE - Prob. 31MPCh. 11.SE - Prob. 32MPCh. 11.SE - Metabolism of S-adenosylhomocysteine (Section...Ch. 11.SE - Reaction of iodoethane with CN- yields a small...Ch. 11.SE - One step in the urea cycle for ridding the body of...Ch. 11.SE - Prob. 36MPCh. 11.SE - Prob. 37MPCh. 11.SE - Propose a mechanism for the following reaction, an...Ch. 11.SE - Prob. 39APCh. 11.SE - The following Walden cycle has been carried out....Ch. 11.SE - Prob. 41APCh. 11.SE - Which reactant in each of the following pairs is...Ch. 11.SE - Prob. 43APCh. 11.SE - Prob. 44APCh. 11.SE - Prob. 45APCh. 11.SE - Prob. 46APCh. 11.SE - Prob. 47APCh. 11.SE - Prob. 48APCh. 11.SE - Propose structures for compounds that fit the...Ch. 11.SE - What products would you expect from the reaction...Ch. 11.SE - Prob. 51APCh. 11.SE - Prob. 52APCh. 11.SE - Prob. 53APCh. 11.SE - Prob. 54APCh. 11.SE - Prob. 55APCh. 11.SE - Order each of the following sets of compounds with...Ch. 11.SE - Order each of the following sets of compounds with...Ch. 11.SE - Prob. 58APCh. 11.SE - Prob. 59APCh. 11.SE - Ethers can often be prepared by SN2 reaction of...Ch. 11.SE - Show the stereochemistry of the epoxide (see...Ch. 11.SE - Prob. 62APCh. 11.SE - In addition to not undergoing substitution...Ch. 11.SE - The tosylate of (2R, 3S)-3-phenyl-2-butanol...Ch. 11.SE - Prob. 65APCh. 11.SE - Prob. 66APCh. 11.SE - Prob. 67APCh. 11.SE - Prob. 68APCh. 11.SE - Prob. 69APCh. 11.SE - (S)-2-Butanol slowly racemizes on standing in...Ch. 11.SE - Reaction of HBr with (R)-3-methyl-3-hexanol leads...Ch. 11.SE - Treatment of 1-bromo-2-deuterio-2-phenylethane...Ch. 11.SE - Prob. 73APCh. 11.SE - Prob. 74APCh. 11.SE - In light of your answer to Problem 11-74, explain...Ch. 11.SE - Prob. 76APCh. 11.SE - Compound X is optically inactive and has the...Ch. 11.SE - When a primary alcohol is treated with...Ch. 11.SE - Prob. 79APCh. 11.SE - Amines are converted into alkenes by a two-step...Ch. 11.SE - The antipsychotic drug flupentixol is prepared by...
Knowledge Booster
Similar questions
- this is an organic chemistry question please answer accordindly!! please post the solution draw the figures on a paper please hand drawn and post, please answer EACH part till the end and dont just provide wordy explanations, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forwardA mixture of 0.412 M C12, 0.544 M F2, and 0.843 M CIF is enclosed in a vessel and heated to 2500 K. C12(g) + F2(g )2CIF(g) Kc = 20.0 at 2500 K Calculate the equilibrium concentration of each gas at 2500 K. [C12] = M [F2] = M [ CIF] =arrow_forwardShow reaction mechanism with explanation. don't give Ai generated solutionarrow_forward
- Don't used Ai solutionarrow_forwardthis is an organic chemistry question please answer accordindly!! please post the solution draw the figures and post, answer the question in a very simple and straight forward manner thanks!!!!! please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures or diagrams, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this kindly solve all parts and draw it not just word explanations!!arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning