
Concept explainers
(a)
Interpretation:
Among the given pair of molecules, the one that is cis isomer and the one that is trans isomer has to be identified.
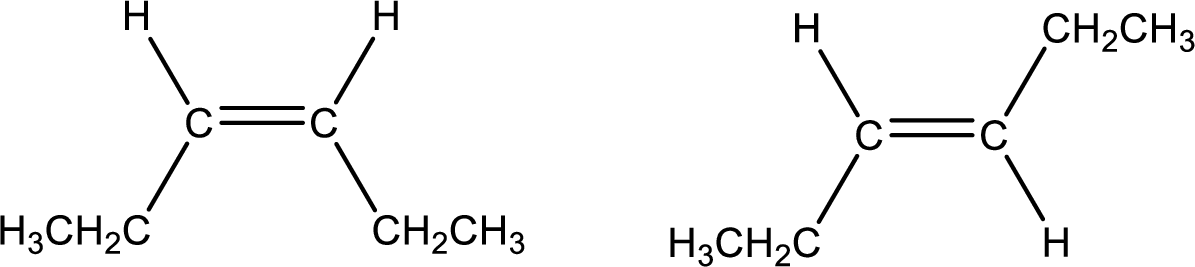
Concept Introduction:
Geometric isomers are obtained for compounds containing carbon‑carbon double bond due to restricted rotation of the double bond. If the groups or atoms that are attached to the carbon atoms present in the double bond are different means, then the molecule is said to have geometric isomerism. Orientation of the group or the atoms that is bonded to the carbon atom will be different resulting in geometric isomers.
If the groups that are attached to the carbon atoms are present on same side of the double bond, then it is known as cis- isomer. If the groups are on opposite side of the double bond means it is known as trans- isomer.
Example:
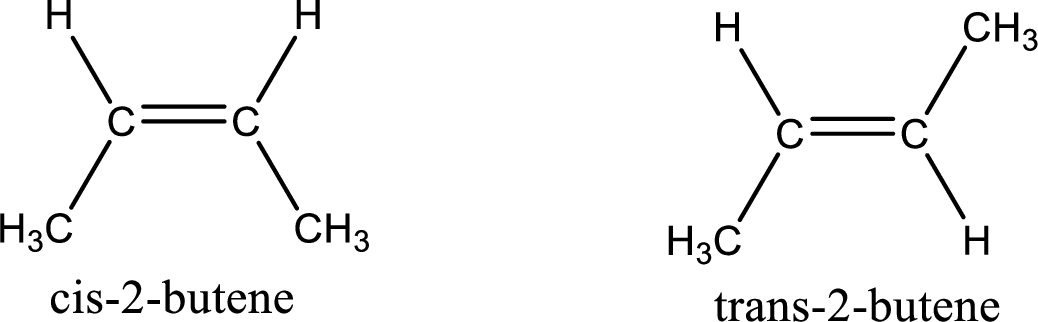
(b)
Interpretation:
Among the given pair of molecules, the one that is cis isomer and the one that is trans isomer has to be identified.

Concept Introduction:
Refer part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
GENERAL,ORGANIC,+BIOCHEMISTRY
- Write the reaction and show the mechanism of the reaction. Include the mechanism for formation of the NO2+ 2. Explain, using resonance structures, why the meta isomer is formed. Draw possible resonance structures for ortho, meta and para.arrow_forwardNonearrow_forward3. A molecular form of "dicarbon", C2, can be generated in gas phase. Its bond dissociation energy has been determined at 599 kJ/mol. Use molecular orbital theory to explain why energy of dissociation for C₂+ is 513 kJ/mol, and that for C2² is 818 kJ/mol. (10 points)arrow_forward
- 9.73 g of lead(IV) chloride contains enough Cl- ions to make ____ g of magnesium chloride.arrow_forward6. a) C2's. Phosphorus pentafluoride PF5 belongs to D3h symmetry group. Draw the structure of the molecule, identify principal axis of rotation and perpendicular (4 points) b) assume that the principal axis of rotation is aligned with z axis, assign symmetry labels (such as a1, b2, etc.) to the following atomic orbitals of the P atom. (character table for this group is included in the Supplemental material). 3s 3pz (6 points) 3dz²arrow_forward2. Construct Lewis-dot structures, and draw VESPR models for the ions listed below. a) SiF5 (4 points) b) IOF4 (4 points)arrow_forward
- 5. Complex anion [AuCl2]¯ belongs to Doh symmetry point group. What is the shape of this ion? (4 points)arrow_forward4. Assign the following molecules to proper point groups: Pyridine N 1,3,5-triazine N Narrow_forward7. a) Under normal conditions (room temperature & atmospheric pressure) potassium assumes bcc lattice. Atomic radius for 12-coordinate K atom is listed as 235 pm. What is the radius of potassium atom under normal conditions? (3 points) b) Titanium metal crystallyzes in hcp lattice. Under proper conditions nitrogen can be absorbed into the lattice of titanium resulting in an alloy of stoichiometry TiNo.2. Is this compound likely to be a substitutional or an interstitial alloy? (Radius of Ti (12-coordinate) is 147 pm; radius of N atom is 75 pm. (3 points)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





