
(a)
Interpretation:
Condensed formula for 2-methyl-2-hexene has to be drawn.
Concept Introduction:
Organic compounds can be drawn from its IUPAC name. Initially, the parent chain is identified from the IUPAC name. After that the carbon chain is drawn with carbon atoms alone. Next step is to add the substituents in the respective positions as indicated in the IUPAC name. Remaining valency of carbon atom is satisfied by adding correct number of hydrogen atoms.
Condensed structural formula is a simplified form of representation of a molecule. This gives the information about all the atoms present in molecule and the atoms are placed in sequential order which gives information about which atom is bonded to other atom.
(a)
Explanation of Solution
Given IUPAC name is 2-methyl-2-hexene. From this the parent
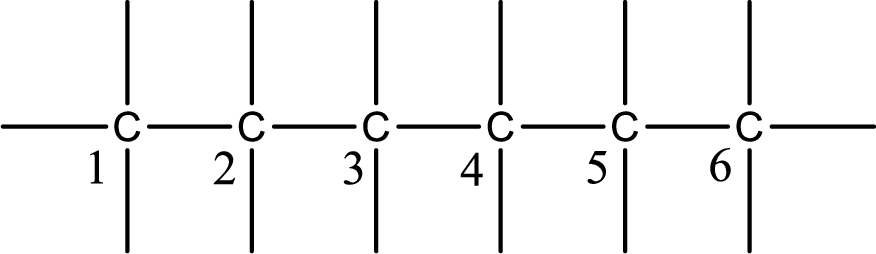
Numbering of each carbon has to be done as shown,
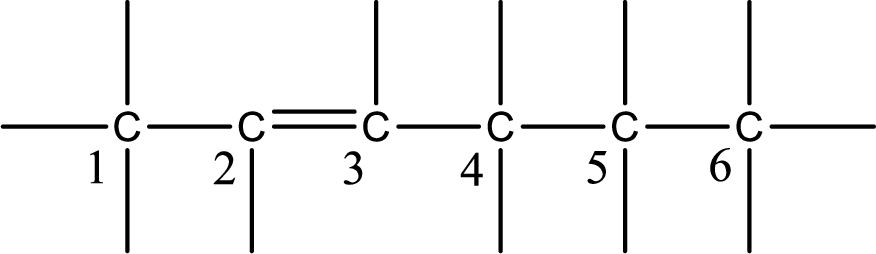
From the name it is understood that a double bond is present between carbon-2 and carbon-3.
Substituent that is present is a methyl group on carbon-2.
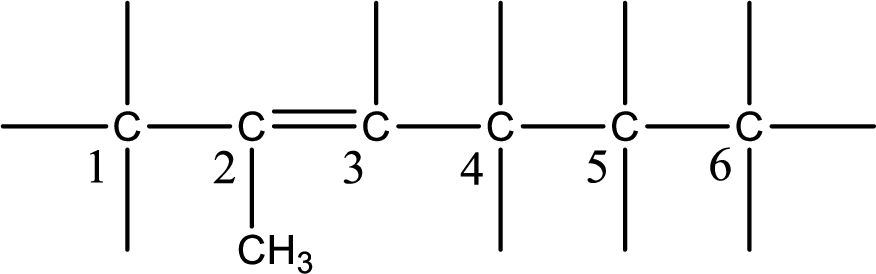
Remaining valency of carbon atom has to be balanced by adding hydrogen atoms.
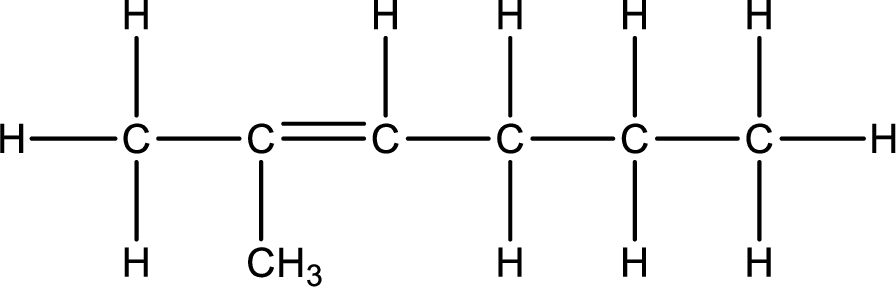
Condensed formula can be obtained as shown below,
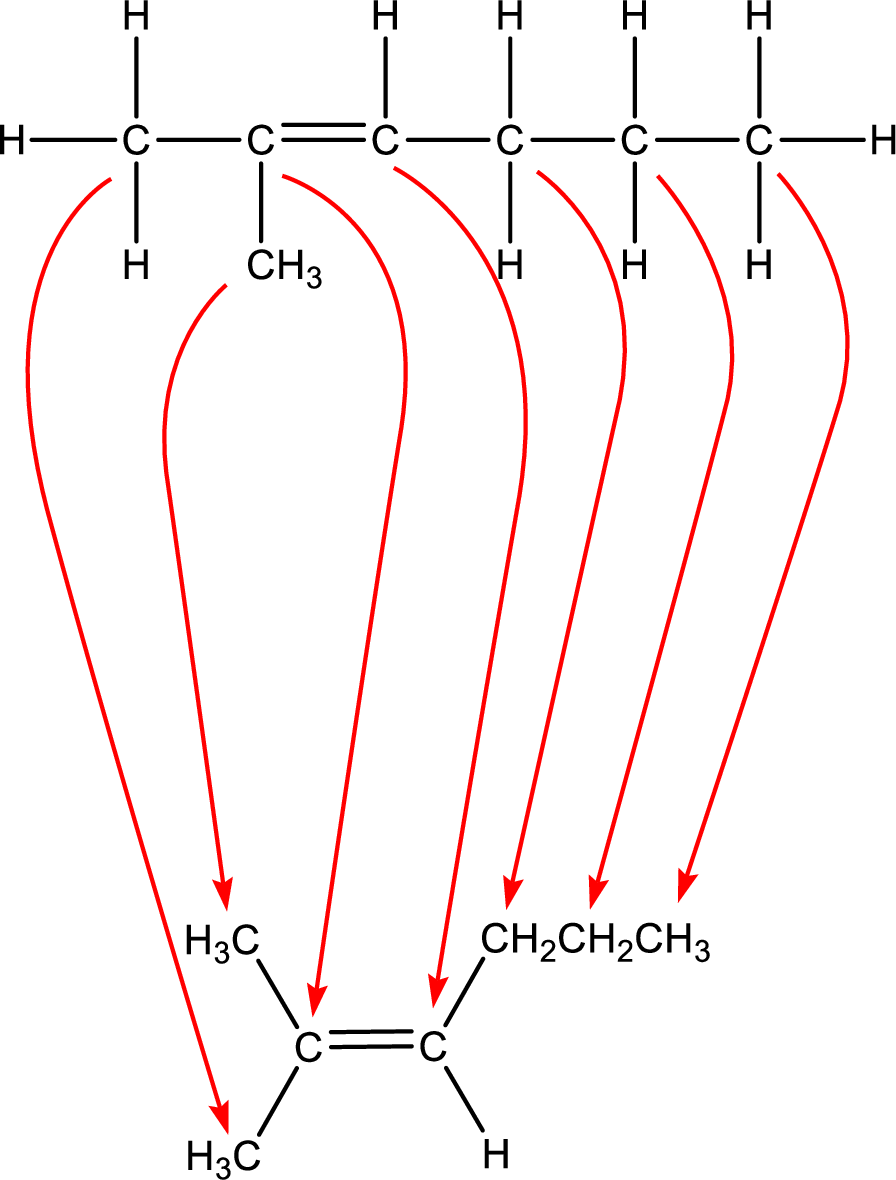
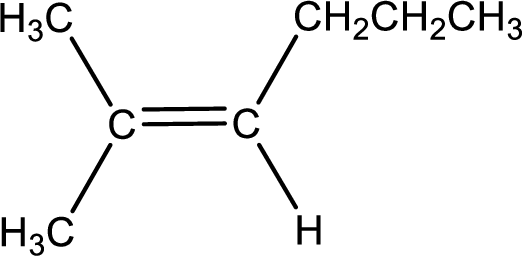
Condensed formula for 2-methyl-2-hexene is drawn as shown.
(b)
Interpretation:
Condensed formula for trans-3-heptene has to be drawn.
Concept Introduction:
Refer part (a).
(b)
Explanation of Solution
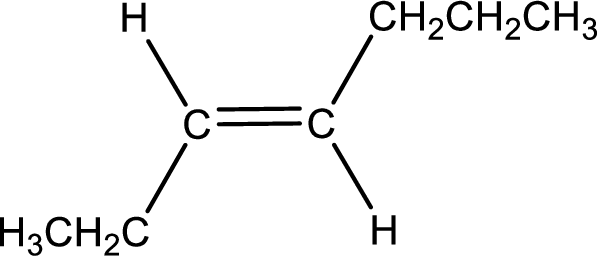
Given IUPAC name is trans-3-heptene. From this the parent alkane is identified as heptane. Heptane contains seven carbon atoms.
Numbering of each carbon has to be done as shown,
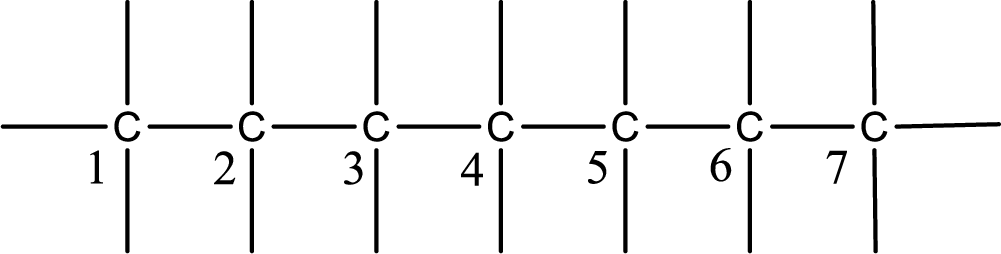
From the name it is understood that a double bond is present between carbon-3 and carbon-4.
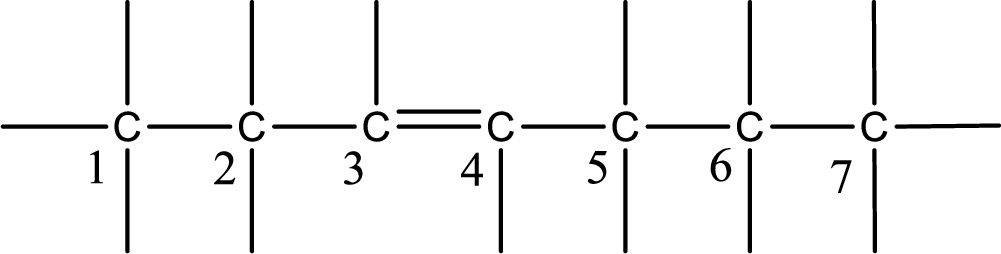
As the stereo information is given as trans-, the hydrogen atom that is bonded to the carbon atoms across the double bond has to be in opposite side. Remaining valency of carbon atom has to be balanced by adding hydrogen atoms.
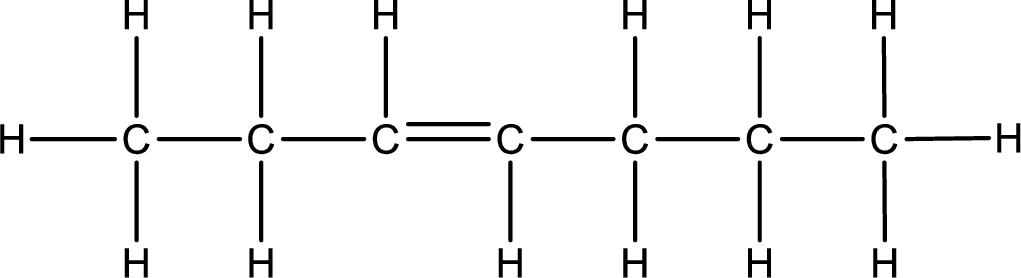
Condensed formula can be obtained as shown below,
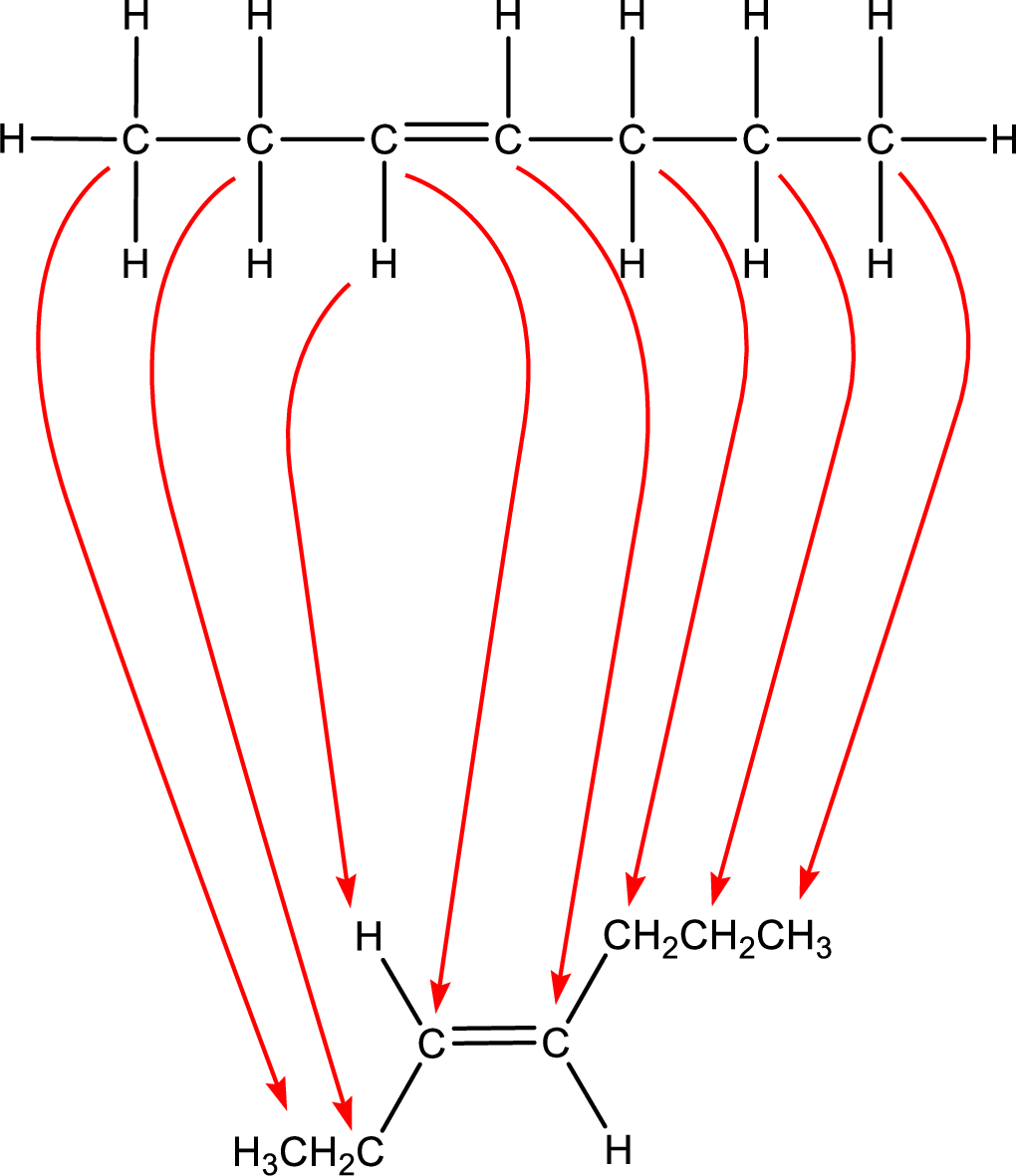
Condensed formula for trans-3-heptene is drawn as shown.
(c)
Interpretation:
Condensed formula for cis-1-chloro-2-pentene has to be drawn.
Concept Introduction:
Refer part (a).
(c)
Explanation of Solution
Given IUPAC name is cis-1-chloro-2-pentene. From this the parent alkane is identified as pentane. Pentane contains five carbon atoms.
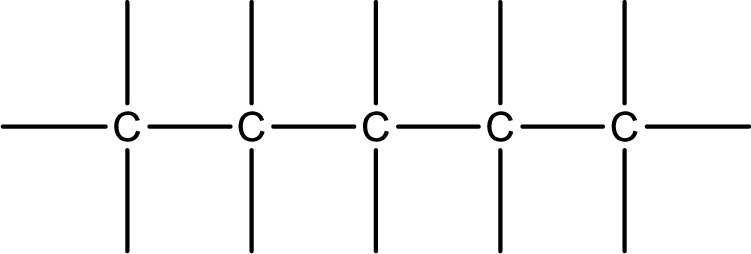
Numbering of each carbon has to be done as shown,
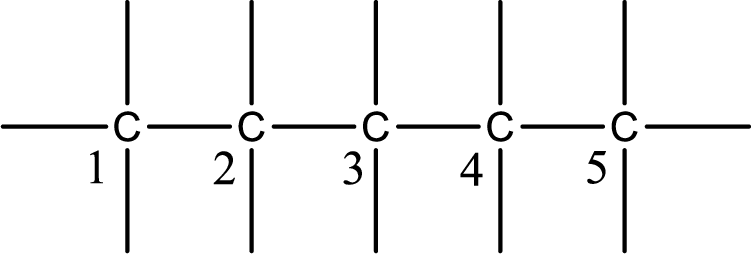
From the name it is understood that a double bond is present between carbon-2 and carbon-3.
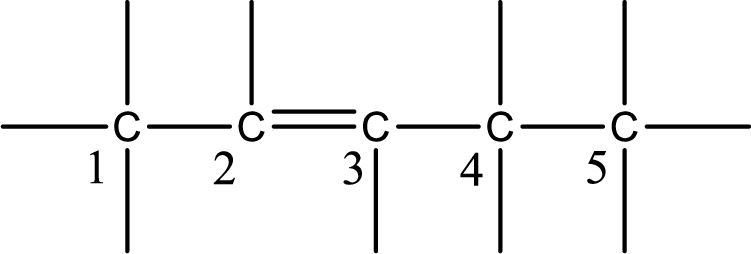
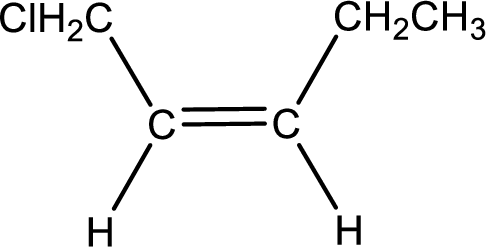
As the stereo information is given as cis-, the hydrogen atom that is bonded to the carbon atoms across the double bond has to be in same side. Subsituents present in the given name is a chlorine atom in on carbon-1. Remaining valency of carbon atom has to be balanced by adding hydrogen atoms.
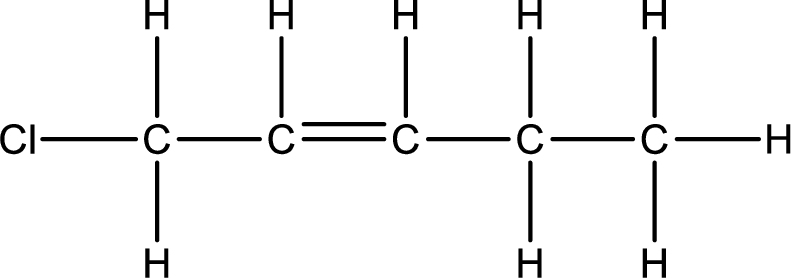
Condensed formula can be obtained as shown below,
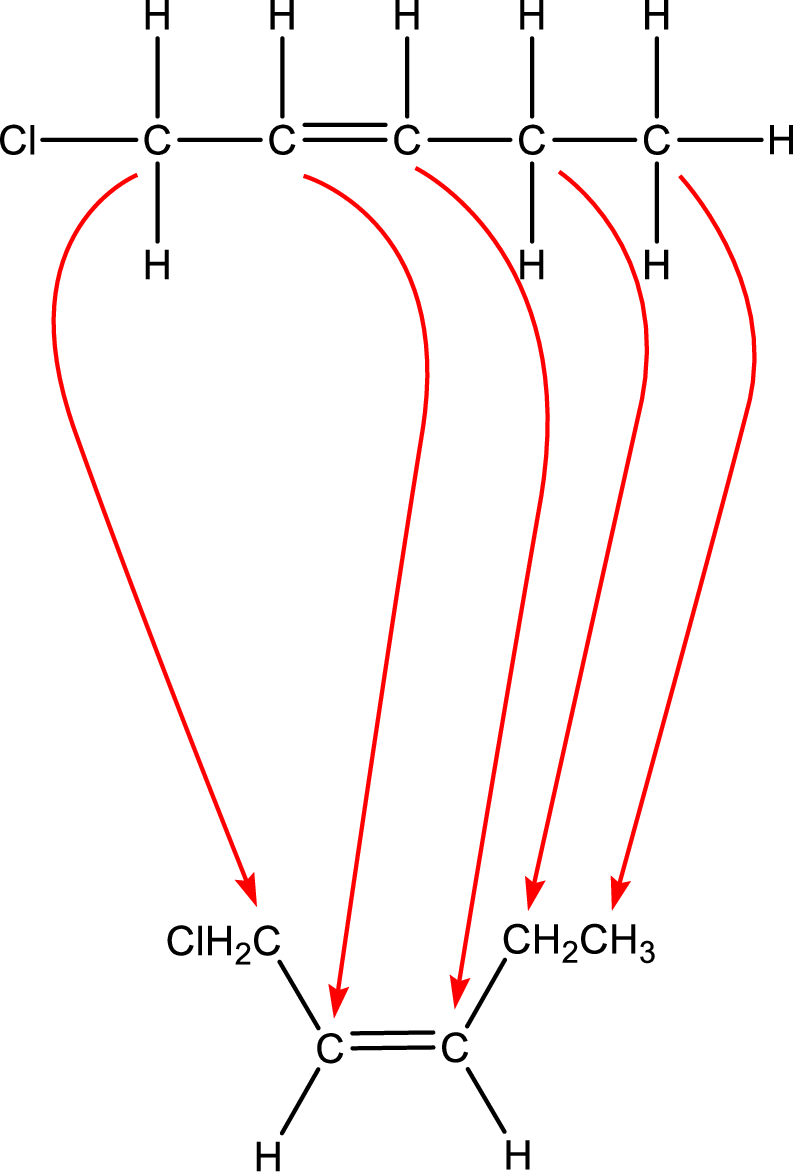
Condensed formula for cis-1-chloro-2-pentene is drawn as shown.
(d)
Interpretation:
Condensed formula for cis-2-chloro-2-methyl-3-heptene has to be drawn.
Concept Introduction:
Refer part (a).
(d)
Explanation of Solution
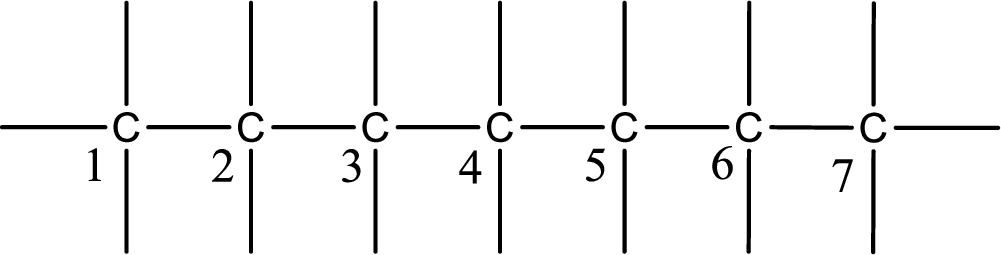
Given IUPAC name is cis-2-chloro-2-methyl-3-heptene. From this the parent alkane is identified as heptane. Heptane contains seven carbon atoms.
Numbering of each carbon has to be done as shown,
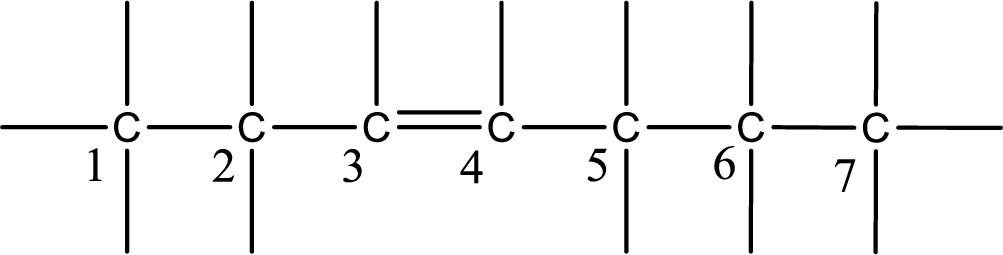
From the name it is understood that a double bond is present between carbon-3 and carbon-4.
As the stereo information is given as cis-, the hydrogen atom that is bonded to the carbon atoms across the double bond has to be in same side. Substituents present in the given name are a chlorine atom in on carbon-2 and methyl group on carbon-2. Remaining valency of carbon atom has to be balanced by adding hydrogen atoms.
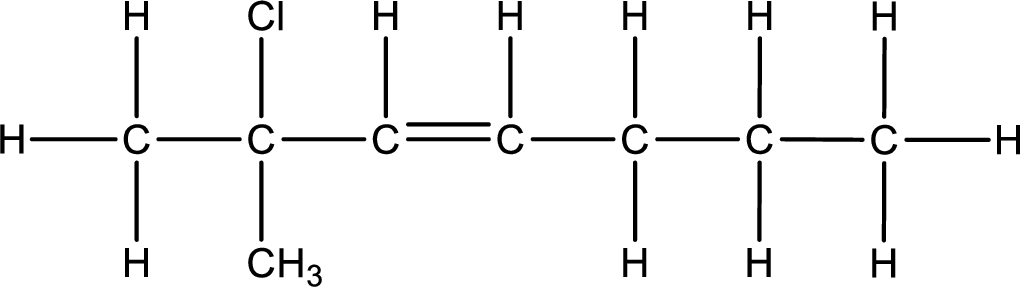
Condensed formula can be obtained as shown below,
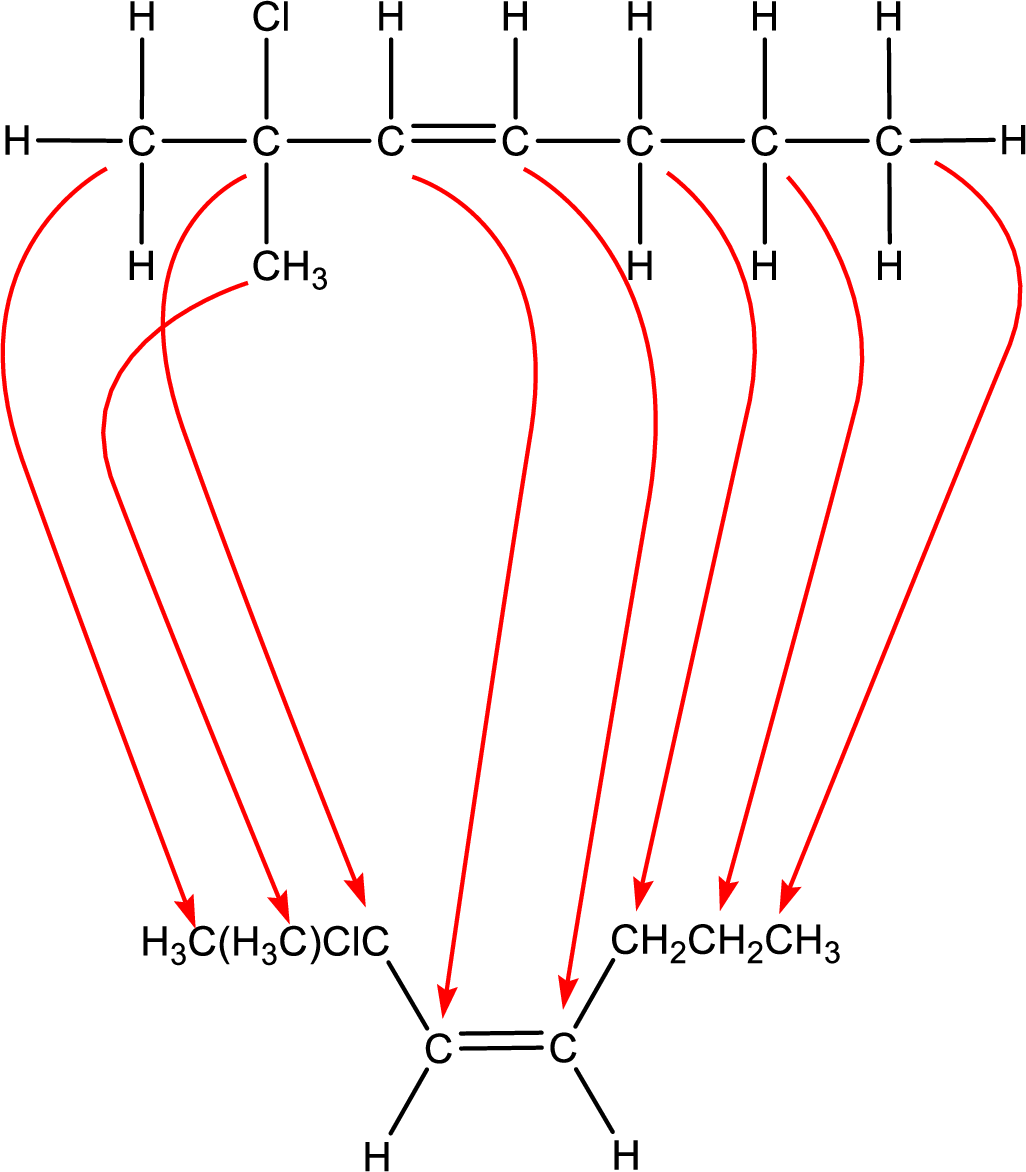
Condensed formula for cis-2-chloro-2-methyl-3-heptene is drawn as shown.
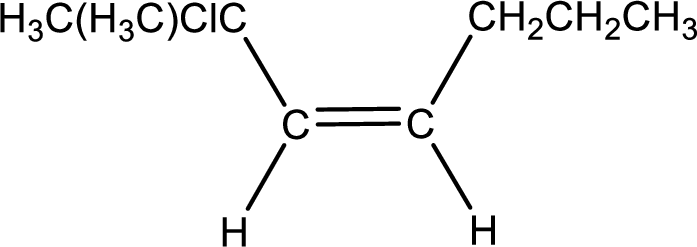
(e)
Interpretation:
Condensed formula for trans-5-bromo-2,6-dimethyl-3-octene has to be drawn.
Concept Introduction:
Refer part (a).
(e)
Explanation of Solution
Given IUPAC name is trans-5-bromo-2,6-dimethyl-3-octene. From this the parent alkane is identified as octane. Octane contains eight carbon atoms.
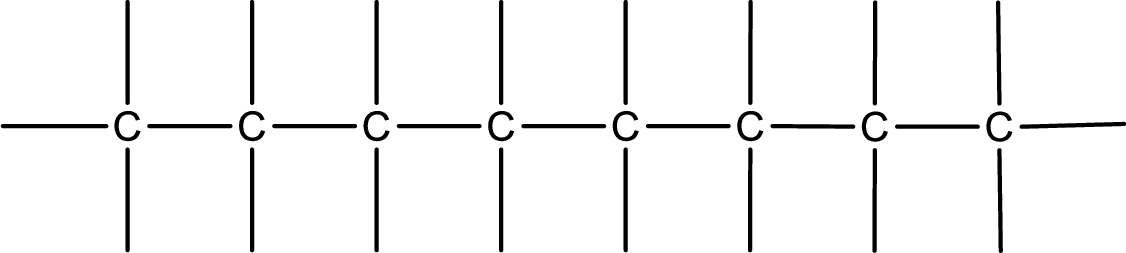
Numbering of each carbon has to be done as shown,
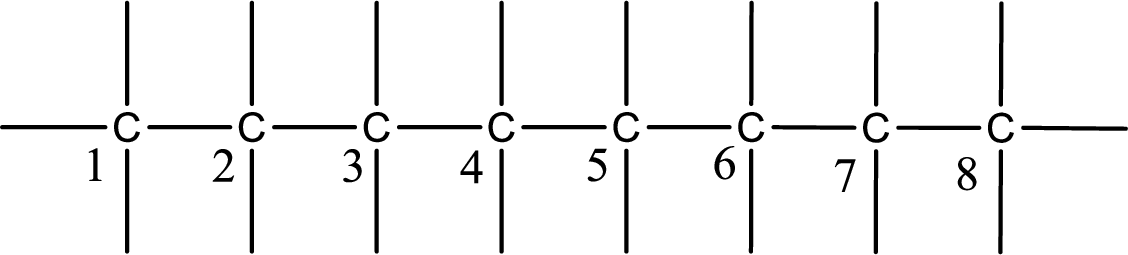
From the name it is understood that a double bond is present between carbon-3 and carbon-4.
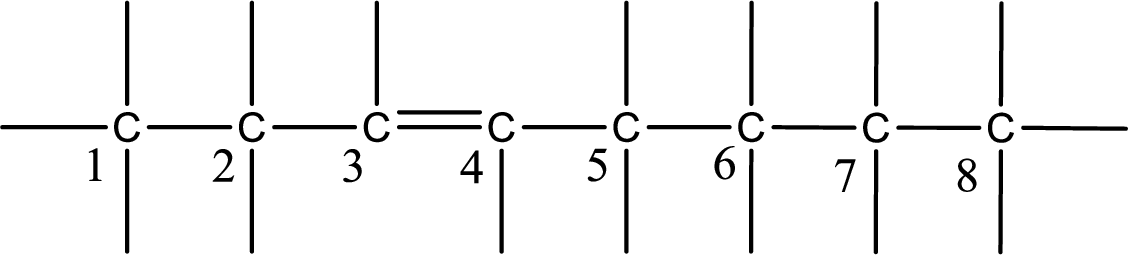
As the stereo information is given as trans-, the hydrogen atom that is bonded to the carbon atoms across the double bond has to be in opposite side. Substituents present in the given name are a bromine atom in on carbon-5, a methyl group on carbon-2, and a methyl group on carbon-6. Remaining valency of carbon atom has to be balanced by adding hydrogen atoms.
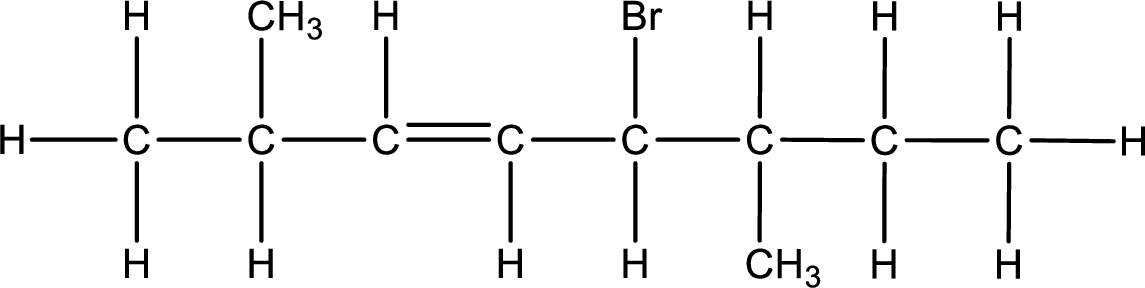
Condensed formula can be obtained as shown below,
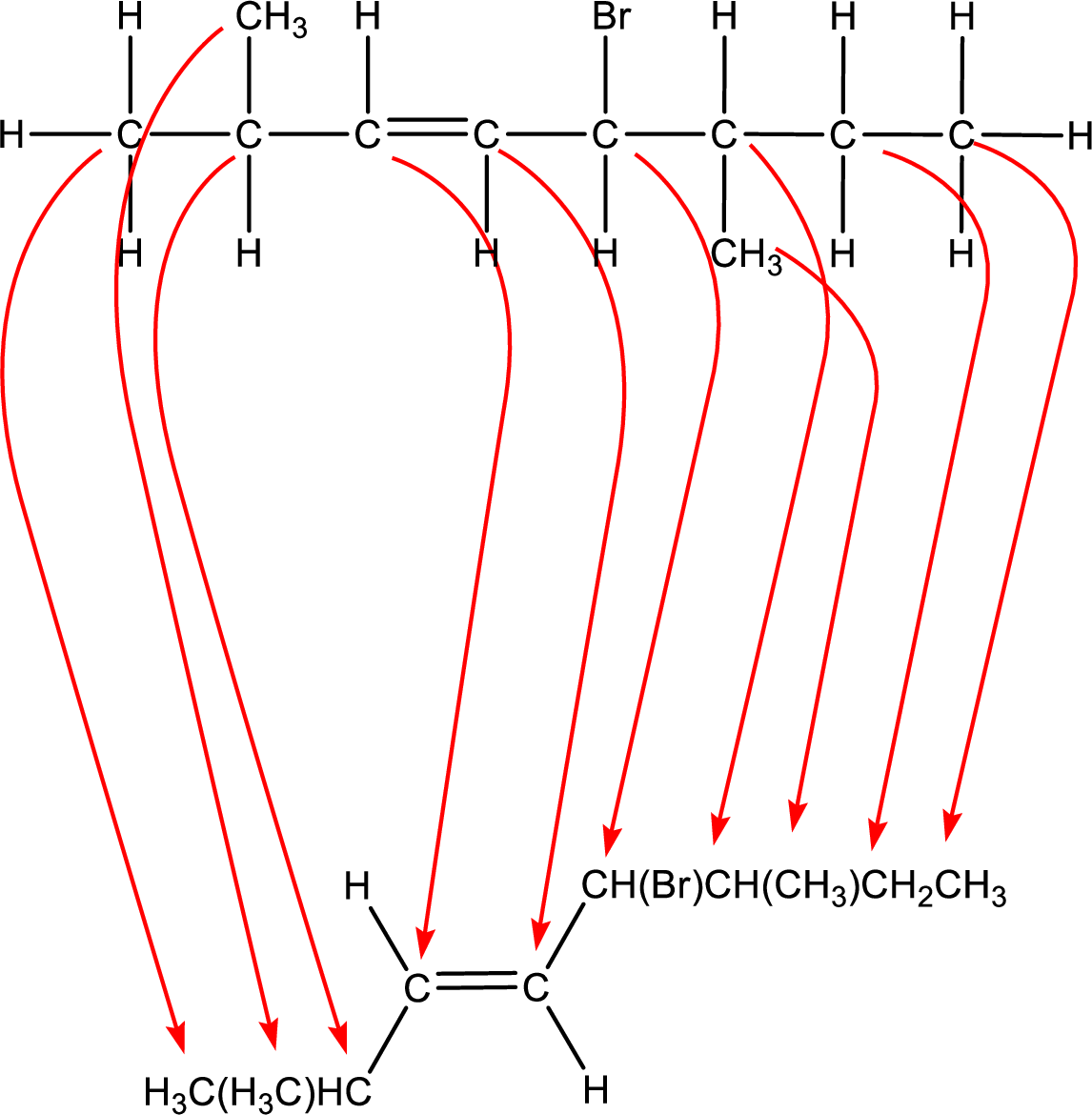
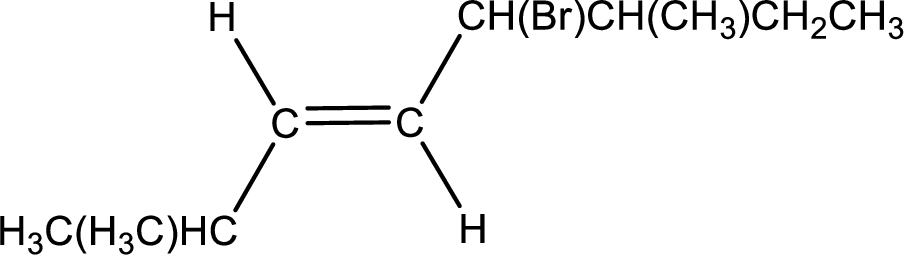
Condensed formula for trans-5-bromo-2,6-dimethyl-3-octene is drawn as shown.
Want to see more full solutions like this?
Chapter 11 Solutions
GENERAL,ORGANIC,+BIOCHEMISTRY
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





