
A commercial refrigerator with refrigerant-134a as the working fluid is used to keep the refrigerated space at −30°C by rejecting its waste heat to cooling water that enters the condenser at 18°C at a rate of 0.25 kg/s and leaves at 26°C. The refrigerant enters the condenser at 1.2 MPa and 65°C and leaves at 42°C. The inlet state of the compressor is 60 kPa and −34°C and the compressor is estimated to gain a net heat of 450 W from the surroundings. Determine (a) the quality of the refrigerant at the evaporator inlet, (b) the refrigeration load, (c) the COP of the refrigerator, and (d) the theoretical maximum refrigeration load for the same power input to the compressor.
FIGURE P11–22
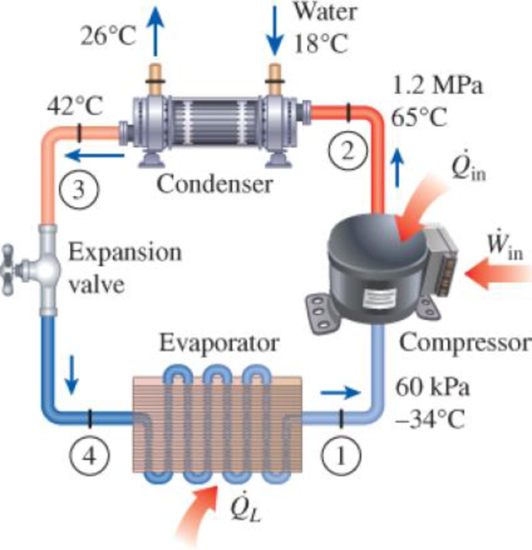
(a)
The quality of the refrigerant at the evaporator inlet.
Answer to Problem 22P
The quality of the refrigerant at the evaporator inlet is
Explanation of Solution
Show the T-s diagram for the refrigeration cycle as in Figure (1).
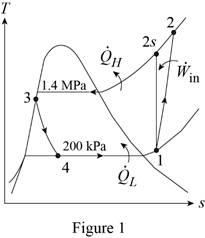
Express specific enthalpy at state 3.
Here, specific enthalpy at saturated liquid and temperature of
Express the quality of the refrigerant at the evaporator inlet.
Here, specific enthalpy at saturated liquid and pressure of
Conclusion:
Perform unit conversion of pressure at state 1 from
Refer Table A-13, “superheated refrigerant-134a”, and write the value of specific enthalpy at state 1
Write the formula of interpolation method of two variables.
Here, the variables denote by x and y is initial temperature and specific enthalpy at state 1 respectively.
Show the specific enthalpy at state 2 corresponding to specific entropy as in Table (1).
|
Initial temperature |
Specific enthalpy at state 1 |
| 227.80 | |
| 240.78 |
Substitute
Perform unit conversion of pressure at state 2 from
Refer Table A-13, “superheated refrigerant-134a”, and write the value of specific enthalpy at state 2
Show the specific enthalpy at state 2 corresponding to temperature as in Table (2).
|
Temperature |
Specific enthalpy at state 2 |
| 289.66 | |
| 300.63 |
Use Excel by taking the values from Table (2), and using Equation (III) to get specific enthalpy at state 2.
Refer Table A-11, “saturated refrigerant 134a-temperature table”, and write the properties corresponding to temperature at state 3 of
Substitute
From Figure (1), write the specific enthalpy at state 3 is equal to state 4 due to throttling process.
Here, specific enthalpy at state 4 is
Refer Table A-12, “saturated refrigerant 134a-pressure table”, and write the properties corresponding to pressure at state 4 of
Substitute
Hence, the quality of the refrigerant at the evaporator inlet is
(b)
The refrigeration load.
Answer to Problem 22P
The refrigeration load is
Explanation of Solution
Express the mass flow rate of the refrigerant from an energy balance on the compressor.
Here, mass flow rate of the water is
Express the rate of heat supplied from the refrigerant.
Express compressor power input.
Here, rate of heat gained by compressor is
Express the refrigeration load.
Conclusion:
Refer Table A-4, “saturated water-temperature table”, and write the initial specific enthalpy of water corresponding to temperature of
Show the initial specific enthalpy of water corresponding to temperature as in Table (3).
|
Temperature |
Initial specific enthalpy of water |
| 15 | 62.982 |
| 18 | |
| 20 | 83.915 |
Use Excel by taking the values from Table (3), and using Equation (III) to get initial specific enthalpy of water.
Refer Table A-4, “saturated water-temperature table”, and write the initial specific enthalpy of water corresponding to temperature of
Show the initial specific enthalpy of water corresponding to temperature as in Table (3).
|
Temperature |
Final specific enthalpy of water |
| 25 | 104.83 |
| 26 | |
| 30 | 125.74 |
Use Excel by taking the values from Table (3), and using Equation (III) to get final specific enthalpy of water.
Substitute
Substitute
Substitute
Substitute
Hence, the refrigeration load is
(c)
The COP of the refrigerator.
Answer to Problem 22P
The COP of the refrigerator is
Explanation of Solution
Express the coefficient of performance of the refrigerator.
Conclusion:
Substitute
Hence, the coefficient of performance of the refrigerator is
(d)
The theoretical maximum refrigeration load.
Answer to Problem 22P
The theoretical maximum refrigeration load is
Explanation of Solution
Express the reversible COP of the refrigerator for the similar temperature limits.
Here, high and low source temperature is
Express the theoretical maximum refrigeration load.
Conclusion:
Substitute
Substitute
Hence, the theoretical maximum refrigeration load is
Want to see more full solutions like this?
Chapter 11 Solutions
Thermodynamics: An Engineering Approach
- H.W6 Determine the largest weight W that can be supported by two wires shown in Fig. P109. The stress in either wire is not to exceed 30 ksi. The cross- sectional areas of wires AB and AC are 0.4 in2 and 0.5 in2, respectively. 50° 30° Warrow_forwardFind equation of motion and natural frequency for the system shown in fig. by energy method. H.W2// For the system Fig below find 1-F.B.D 2-Eq.of motion 8wn 4-0 (5) m. Jo marrow_forward2. Read the following Vernier caliper measurements. (The scales have been enlarged for easier reading.) The Vernier caliper is calibrated in metric units. (a) 0 1 2 3 4 5 سلسلسله (b) 1 2 3 4 5 6 سلسل (c) 1 23456 (d) 1 2 3 4 5 6 سلسلسarrow_forward
- Explain why on the interval 0<x<1000 mm and 1000<x<2000mm, Mt is equal to positive 160 Nm, but at x= 0mm and x=1000mm Mt is equal to -160 Nm (negative value!). What is the reason for the sign change of Mt?arrow_forward20 3. 2-233 2520 Тр Gears 1079 A pair of helical gears consist of a 20 teeth pinion meshing with a 100 teeth gear. The pinion rotates at Ta 720 r.p.m. The normal pressure angle is 20° while the helix angle is 25°. The face width is 40 mm and the normal module is 4 mm. The pinion as well as gear are made of steel having ultimate strength of 600 MPa and heat treated to a surface hardness of 300 B.H.N. The service factor and factor of safety are 1.5 and 2 respectively. Assume that the velocity factor accounts for the dynamic load and calculate the power transmitting capacity of the gears. [Ans. 8.6 kWarrow_forward4. A single stage helical gear reducer is to receive power from a 1440 r.p.m., 25 kW induction motor. The gear tooth profile is involute full depth with 20° normal pressure angle. The helix angle is 23°, number of teeth on pinion is 20 and the gear ratio is 3. Both the gears are made of steel with allowable beam stress of 90 MPa and hardness 250 B.H.N. (a) Design the gears for 20% overload carrying capacity from standpoint of bending strength and wear, (b) If the incremental dynamic load of 8 kN is estimated in tangential plane, what will be the safe power transmitted by the pair at the same speed?arrow_forward
- Determine the stress in each section of the bar shown in Fig. when subjected to an axial tensile load shown in Fig. The central section is 30 mm hollow square cross- section; the other portions are of circular section, their diameters being indicated What will be the total deformation of the bar? For the bar material E = 210GPa. 20mi О 30mm 30mmm 2.6 15mm 30kN 1 2 10kN - 20kN 3 -329 91mm 100mm 371mmarrow_forwardCalculate the load that will make point A move to the left by 6mm, E=228GPa. The diameters of the rods are as shown in fig. below. 2P- PA 80mm B 200mm 2P 0.9m 1.3m.arrow_forwardIf the rods are made from a square section with the dimension as shown. Calculate the load that will make point A move to the left by 6mm, E=228GPa. 2P- P A 80mm B 200mm 2P 0.9m 1.3marrow_forward
- 3. 9. 10. The centrifugal tension in belts (a) increases power transmitted (b) decreases power transmitted (c) have no effect on the power transmitted (d) increases power transmitted upto a certain speed and then decreases When the belt is stationary, it is subjected to some tension, known as initial tension. The value of this tension is equal to the (a) tension in the tight side of the belt (b) tension in the slack side of the belt (c) sum of the tensions in the tight side and slack side of the belt (d) average tension of the tight side and slack side of the belt The relation between the pitch of the chain (p) and pitch circle diameter of the sprocket (d) is given by 60° (a) p=d sin (c) p=d sin (120° T where T Number of teeth on the sprocket. 90° (b) p=d sin T 180° (d) p=d sin Tarrow_forwardOBJECTIVE TYPE QUESTIONS 1. The maximum fluctuation of energy is the 2. (a) sum of maximum and minimum energies (b) difference between the maximum and minimum energies (c) ratio of the maximum energy and minimum energy (d) ratio of the mean resisting torque to the work done per cycle In a turning moment diagram, the variations of energy above and below the mean resisting torque line is called (a) fluctuation of energy (b) maximum fluctuation of energy (c) coefficient of fluctuation of energy (d) none of the above Chapter 16: Turning Moment Diagrams and Flywheel 611 The ratio of the maximum fluctuation of speed to the mean speed is called 3. (a) fluctuation of speed (c) coefficient of fluctuation of speed 4. (b) maximum fluctuation of speed (a) none of these The ratio of the maximum fluctuation of energy to the.......... is called coefficient of fluctuation of energy. (a) minimum fluctuation of energy (b) work done per cycle The maximum fluctuation of energy in a flywheel is equal to 5.…arrow_forwardOBJECTIVE TYPE QUESTIONS 1. The velocity ratio of two pulleys connected by an open belt or crossed belt is 2. (a) directly proportional to their diameters (b) inversely proportional to their diameters (c) directly proportional to the square of their diameters (d) inversely proportional to the square of their diameters Two pulleys of diameters d, and d, and at distance x apart are connected by means of an open belt drive. The length of the belt is (a)(d+d₁)+2x+ (d₁+d₂)² 4x (b)(d₁-d₂)+2x+ (d₁-d₂)² 4x (c)(d₁+d₂)+ +2x+ (d₁-d₂)² 4x (d)(d-d₂)+2x+ (d₁ +d₂)² 4x 3. In a cone pulley, if the sum of radii of the pulleys on the driving and driven shafts is constant, then (a) open belt drive is recommended (b) cross belt drive is recommended (c) both open belt drive and cross belt drive are recommended (d) the drive is recommended depending upon the torque transmitted Due to slip of the belt, the velocity ratio of the belt drive 4. (a) decreases 5. (b) increases (c) does not change When two pulleys…arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





