
Concept explainers
(a)
Interpretation:
The Lewis structure of the given compound
Concept Introduction:
Lewis structure:
The representation of valence shell electrons around the atom is known as Lewis structure or Lewis dot structure. Electrons are represented as a dot in Lewis structures, a single dot represents unpaired electron and paired of dots represents paired electrons.
(a)
Explanation of Solution
Given compound,
The total number of valence electrons in
The skeletal structure of
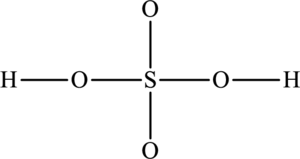
The total number of 12 electrons are used for bonding. Remaining 22 electrons can be distributed over oxygen atoms to get the octet.
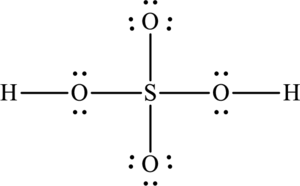
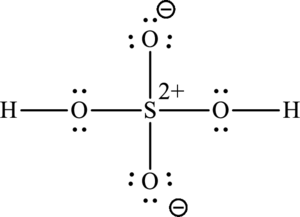
The Lewis structure of compound showing charges can be given as follows:
(b)
Interpretation:
The Lewis structure of the given compound
Concept Introduction:
Refer part (a)
(b)
Explanation of Solution
Given compound,
The total number of valence electrons in
The skeletal structure of
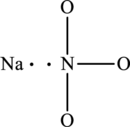
The total number of 8 electrons are used for bonding. Remaining 16 electrons can be distributed over oxygen atoms to get the octet.
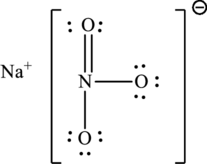
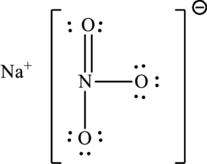
The Lewis structure of compound showing charges can be given as follows:
(c)
Interpretation:
The Lewis structure of the given compound
Concept Introduction:
Refer part (a)
(c)
Explanation of Solution
Given compound,
The total number of valence electrons in
The skeletal structure of
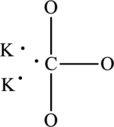
The total number of 6 electrons are used for bonding. Remaining 18 electrons can be distributed over oxygen atoms to get the octet.
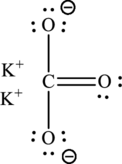
The Lewis structure of compound showing charges can be given as follows:
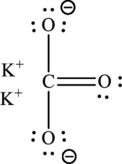
(d)
Interpretation:
The Lewis structure of the given compound
Concept Introduction:
Refer part (a)
(d)
Explanation of Solution
Given compound,
The total number of valence electrons in
The skeletal structure of
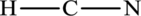
The total number of four electrons are used for bonding. Remaining 6 electrons can be distributed over nitrogen and carbon atoms to get the octet.
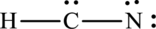
The Lewis structure of compound showing charges can be given as follows:
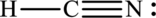
(e)
Interpretation:
The Lewis structure of the given compound
Concept Introduction:
Refer part (a)
(e)
Explanation of Solution
Given compound,
The total number of valence electrons in
The skeletal structure of
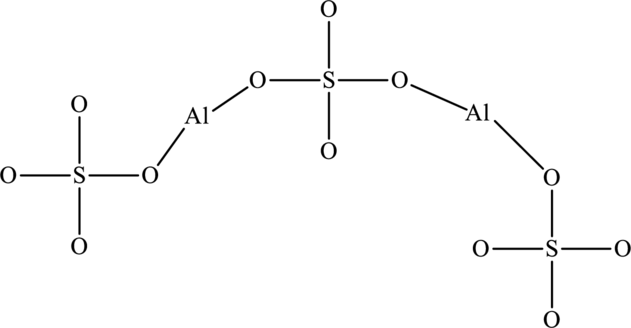
The total number of 32 electrons are used for bonding. Remaining 64 electrons can be distributed over oxygen atoms to get the octet.
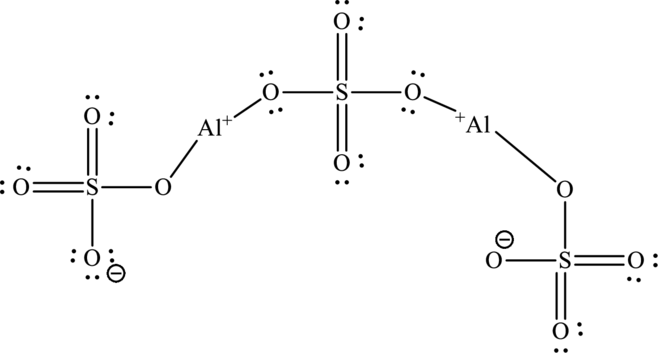
The Lewis structure of compound showing charges can be also be represented as follows:
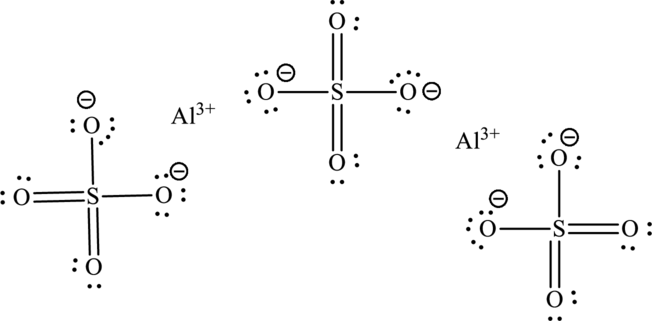
(f)
Interpretation:
The Lewis structure of the given compound
Concept Introduction:
Refer part (a)
(f)
Explanation of Solution
Given compound,
The total number of valence electrons in
The skeletal structure of
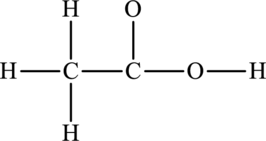
The total number of 14 electrons are used for bonding. Remaining 10 electrons can be distributed over oxygen and carbon atoms to get the octet.
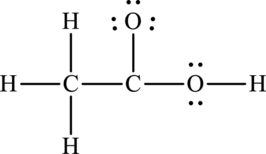
Here octet of carbon attached to oxygen is not completed. Therefore, the Lewis structure of compound can be given as follows:
Want to see more full solutions like this?
Chapter 11 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
- Draw the Lewis structures for each of the following ions or molecules. Give the number of electrons in each species. Remember to enclose ion s in square brackets with the charge as a superscript outside the right bracket. (a) SO 2 (b) XeO 2 F 2 (Xe is the central atom) (c) ClF 3 (d) ClO 2 F (Cl is the central atom) (e) BrO 4 -arrow_forwardPredict which of these compounds are ionic and which are covalent.(A) Ca3N2(B) Li2CO3(C) PCl5(D) NaOH(E) CH4(F) MgOarrow_forwardSolid rocket fuel utilizes the oxidation of methyl hydrazine by dinitrogen tetroxide for propulsion: [UNBALANCED] O2NNO2(l) + (CH3)HNNH2(l) à H2O(g) + N2(g) + CO2(g) (A) Balance the equation (integers). (B) Draw the Lewis dot structures for each chemical, methyl hydrazine and dinitrogen tetroxide are given. Show all electrons. (C) Use the following bond energies to estimate DH for the reaction as balanced in (A). Bond dissociation energies (kJ/mol) C - H 413 C - C 347 C - O 358 N - N 160 C - N 305 O - H 467 C = C 614 C = O 799 N = N 418 C = N 615 N - H 391 C ≡ C 839 O - O 146 N ≡ N 941 C ≡ N 891 N - O 201 N = O 607 O = O…arrow_forward
- Draw the Lewis structures for each of the following ions or molecules. Give the number of electrons in each species. Remember to enclose e ions in square brackets with the charge as a superscript outside the rightbracket.(a) SO 2 F 2 (S is the central atom) (b) PCl 3 (c) BrOF 3 (Br is the central atom) (d) IF 5 (e) IO 2 -arrow_forward- Classify each compound as ionic or molecular. If it is ionic, determine whether the metal forms only one type of ion or more than one type of ion. (a) CoCl2 (b) CF4 (c) BaSO4 (d) NOarrow_forwardName each ionic compound. In each of these compounds,the metal forms only one type of ion. (a) CsCl (b) SrBr2 (c) K2O (d) LiFarrow_forward
- The atomic number of sulfur is 16. Sulfur combines withhydrogen by covalent bonding to form a compound, hydrogensulfide. Based on the number of valence electrons in a sulfuratom, predict the molecular formula of the compound.(A) HS(B) HS2(C) H2S(D) H4Sarrow_forwardAn element X reacts with oxygen to form XO2 and with chlorineto form XCl4. XO2 is a white solid that melts at high temperatures(above 1000 °C). Under usual conditions, XCl4 is acolorless liquid with a boiling point of 58 °C. (a) XCl4 reactswith water to form XO2 and another product. What is thelikely identity of the other product? (b) Do you think thatelement X is a metal, nonmetal, or metalloid?arrow_forwardMany chemical names are similar at first glance. Give the for-mulas of the species in each set: (a) ammonium ion and ammo-nia; (b) magnesium sulfide, magnesium sulfite, and magnesiumsulfate; (c) hydrochloric acid, chloric acid, and chlorous acid; (d) cuprous bromide and cupric bromidearrow_forward
- Predict which ions are stable: (a) Br2- (b) C4- (c) Ca+ (d) Ar+ (e) Na+ (f) Cs+arrow_forwardAnswer true or false. (a) The name of a binary ionic compound consists of the name of the positive ion followed by the name of the negative ion. (b) In naming binary ionic compounds, it is necessary to state the number of each ion present in the compound. (c) The formula of aluminum oxide is Al2 O3 . (d) Both copper(II) oxide and cupric oxide are acceptable names for CuO. (e) The systematic name for Fe2 O3 is iron(II) oxide. (f) The systematic name for FeCO3 is iron carbonate. (g) The systematic name for NaH2PO4 is sodium di- hydrogen phosphate. (h) The systematic name for K2HPO4 is dipotassium hydrogen phosphate. (i) The systematic name for Na2O is sodium oxide. (j) The systematic name for PCl3 is potassium chloride. (k) The formula of ammonium carbonate is NH4CO3. 39. (a) A covalent bond is formed between two atoms whose difference in electronegativity is less than 1.9. (b) If the difference in electronegativity between two atoms is zero (they have identical electronegativ- ities),…arrow_forwardAn element X reacts with oxygen to form XO2 and with chlorineto form XCl4. XO2 is a white solid that melts at high temperatures(above 1000 °C). Under usual conditions, XCl4 is acolorless liquid with a boiling point of 58 °C. (a) XCl4 reactswith water to form XO2 and another product. What is thelikely identity of the other product? (b) Do you think thatelement X is a metal, nonmetal, or metalloid? (c) By using asourcebook such as the CRC Handbook of Chemistry and Physics,try to determine the identity of element X.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
