
Concept explainers
Interpretation:
The reason for which fluorine is given importance in terms of electronegativity and what combination of atoms on periodic table form the ionic bond have to be identified.
Concept Introduction:
Lewis structure:
The representation of valence shell electrons around the atom is known as Lewis structure or Lewis dot structure. Electrons are represented as a dot in Lewis structures, a single dot represents unpaired electron and paired of dots represents paired electrons.
Explanation of Solution
Given,
The molar mass of the compound is
The percentage composition of carbon is
The mass of carbon can be calculated as shown below.
The percentage composition of chlorine is
The mass of carbon is
The mass of chlorine is
The empirical formula can be calculated as follows:
The empirical formula of the compound is
The empirical formula mass is sum of the mass of carbon and two chlorine atoms.
The molar mass of the compound is
The molecular formula is calculated as follows:
Here it is found that empirical formula is twice molecular formula. Therefore the molecular formula of the compound is
The Lewis structure of
The total number of valence electrons in
The four chlorine atoms are bonded to two carbon atoms. Write skeletal structure representing this and place two electrons between each carbon and chlorine atom.
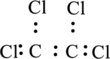
Here
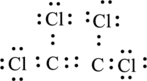
Here all the atoms form octet. Therefore, Lewis structure of
Want to see more full solutions like this?
Chapter 11 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
- An ionic compound has the following composition (by mass): Mg, 10.9%; Cl, 31.8%; O, 57.3%. What are the formula and name of the compound? Write the Lewis formulas for the ions.arrow_forwardWrite the Lewis structure for nitrosyl fluoride, FNO. Using only a periodic table, identify (a) which is the longer bond. (b) which is the stronger bond. (c) which is the more polar bond.arrow_forwardDraw Lewis structures for the following polyatomic ions. a. OH b. BeH42 c. AlCl4 d. NO3arrow_forward
- A polyatomic ion is composed of C, N, and an unknown element X. The skeletal Lewis structure of this polyatomic ion is [XCN]. The ion X2 has an electron configuration of [Ar]4s23d104p6. What is element X? Knowing the identity of X, complete the Lewis structure of the polyatomic ion, including all important resonance structures.arrow_forwardDraw Lewis symbols for the following ions. a. O2 b. S2 c. Si4 d. Clarrow_forwardMany monatomic ions are found in seawater, including the ions formed from the following list of elements. Write the Lewis symbols for the monatomic ions formed from the following elements: (a) Cl (b) Na (c) Mg (d) Ca (e) K (f) Br (g) Sr (h) Farrow_forward
- Without actually drawing the Lewis structure, determine how many valence electrons are available for covalent bonding in each of the following molecules. a. SiH4 b. NCl3 c. H2S d. Cl2Oarrow_forwardA gaseous compound has the following composition by mass: C, 25.0%; H, 2.1%; F, 39.6%; 0. 33.3%. Its molecular mass is 96.0 amu. Write the Lewis formula for the molecule.arrow_forwardSuccessive substitution of F atoms for H atoms in the molecule CH4 produces the molecules CH3F, CH2F2, CHF3, and CF4. a. Draw Lewis structures for each of the five molecules. b. Using VSEPR theory, predict the geometry of each of the five molecules. c. Specify the polarity (polar or nonpolar) for each of the five molecules.arrow_forward
- Methanol, H3COH, is used as the fuel in some race cars. Ethanol, C2H5OH, is used extensively as motor fuel in Brazil. Both methanol and ethanol produce CO2 and H2O when they burn. Write the chemical equations for these combustion reactions using Lewis structures instead of chemical formulas.arrow_forwardWhat possible error(s) exist in the Lewis structure (assume we are trying to represent the best possible Lewis structure for the NO₂S ion knowing N is the central atom in this polyatomic ion)? [:ö==S: N= CO :O: The best structure would have double bond and two lone pairs on each oxygen atom and a single bond with three lone pairs on the sulfur. There are no errors. This is the best possible structure. The Lewis structure above does not minimize formal charges, thus is the not the best possible structure. The nitrogen atom has an expanded octet, and this structure is impossible. The Lewis structure contains the wrong number of electrons, thus this structure is impossible.arrow_forwardDraw the Lewis structures for all seven diatomic elements (H2, N2, O2,F2, Cl2,Br2, I2) . Even though Br and I are not in the first 3 periods it is useful to consider them here.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





